Nelson1 project protocol
Effects of dietary restriction on body composition and lifespan: life extension and life shortening in 44 ILSXISS recombinant inbred strains of mice (2010)
Nelson JF, Johnson TEWith: Liao C-Y, Rikke BA, Gelfond J, Diaz V
Project protocol — Contents
Workflow and sampling
Workflow and sampling
Equipment and supplies
Reagents and solutions
Procedures
Data
ReferencesWorkflow
Procedure performed
- Balance scale
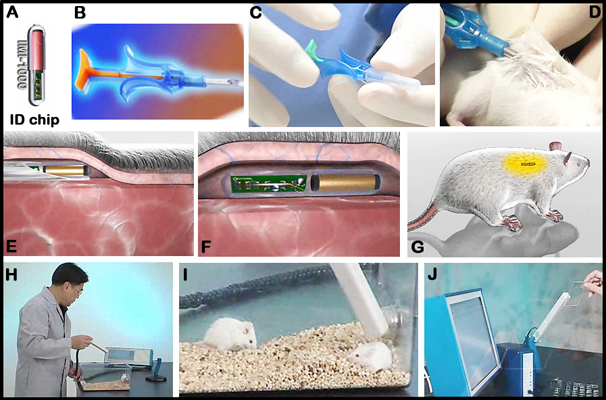
- ID implantable chips: AVID Microchip ID Systems (Folsom, LA, USA; catalog AVID3002)
- Transponders (rice-grain-size, ~0.1 g) (Bio Medic Data Systems, Seaford, DE) for mouse identification
- QMR machine utilizing NMR (EchoMRI; Echo Medical System, Houston TX USA)
- Plastic restrainer tubes for QMR machine
- Superglue (Loctite gel, purchased locally, or Nexaband S/C, purchased from Abbott Laboratories, North Chicago, IL, USA).
- Harlan-Teklad 7912 diet (>19% crude protein, >5% crude fat, <5% crude fiber); nutrient requirements for laboratory mice and nutrient composition of Harlan-Teklad 7912:
Diet Composition Mouse Requirementa Harlan-Teklad 7912 Calcium (%) Phosphorus (%) Sodium (%) Chlorine (%) Potassium (%) Magnesium (%) Iron (mg/kg) Manganese (mg/kg) Zinc (mg/kg) Copper (mg/kg) Iodine (mg/kg) Cobalt (mg/kg) Selenium (mg/kg) Vitamin A (IU/g) Vitamin D3 (IU/g) Vitamin E (IU/kg) Choline (mg/kg) Niacin/Vitamin B3 (mg/kg) Pantothenic Acid/Vitamin B5 (mg/kg) Pyridoxine/Vitamin B6 (mg/kg) Thiamine/Vitamin B1 (mg/kg) Menadione (mg/kg) Folic Acid/Vitamin B9 (mg/kg) Biotin (mg/kg) Vitamin B12 (mcg/kg) a National Research Council requirements for mice (Nutrient Requirements for Laboratory Animals, 1995).
ND = not determined.I. Mouse identification
a. For accurate and reliable identification, transponders are used (see above Figure).
b. Each mouse is briefly anesthetized for 1â2 min with isoflurane inhalation administered either via nose cone or with an instrument designed for small animal anesthesia.
c. An electronic ID chip is then implanted using a sterile syringe beneath the dorsal skin between the shoulder blades.
d. A portion of the distal tail (1 cm) is sampled and frozen for later analysis of DNA polymorphisms.
e. The injection wound is closed with a drop of superglue before each mouse is allowed to recover from the anesthesia.
f. Mice without transponders are given a left-ear or right-ear punch for identification.Submitting PI notes: "An ANOVA of lifespan using diet, strain, and transponder as between group factors indicated no main effect of the transponders (p=0.22) and no interaction with diet or strain or both (p>0.8)."
II. Longevity feeding
a. At 2â5 mo of age mice are fed ad libitum (AL) or 40% diet restriction (DR; 60% of strain-specific AL intake).
b. DR rations are calculated on the basis of AL food intake measured weekly for each strain and adjusted for wastage (Ikeno et al., 2005). To measure food consumption, the amount of chow removed from the cage hopper and the spillage (the chow on the bottom of the cage) are weighed weekly. Actual food consumed is calculated by subtracting the spillage from the chow removed from the hopper.
c. DR mice are fed approximately 1h before the start of the dark phase of a 12-hour light/dark cycle (lights on at 0050h).
d. Rations are weighed to the nearest 0.1 g and delivered manually to the bottom of the cage, rather than the food hopper, to make gnawed pellets easier to grasp and more accessible.
e. The AL and DR cages are maintained side by side in alternating columns of AL and DR to minimize the potential for shelf-level variation in AL food intake.
f. Because AL mice exhibit a substantial decline in their food intake with age, the DR rations are kept constant and fixed by 12 mo of age.III. Body weight and body composition
a. At 15-17 mo of age, mice are immobilized using a plastic restrainer tubes (no sedation) placed in the QMR machine.
b. Whole-body-composition analysis is conducted, and AL and DR mice are analyzed over the same time period.
c. From scanned images, total body fat mass and lean mass are calculated.
d. Body weight is recorded using a balance scale.IV. Longevity mouse monitoring
a. When half of the mice from the same diet and strain die, the remaining mice are consolidated in a single cage in keeping with the original cage density (5 for AL, 6 for DR).
b. Mice are examined at least daily for signs of ill health and any mouse found dead is noted.
c. Moribund mice are euthanized (a mouse is considered severely moribund if it exhibits more than one of the following clinical signs: (i) inability to eat or drink; (ii) severe lethargy, as indicated by reluctance to move when gently prodded with forceps; (iii) severe balance or gait disturbance; (iv) rapid weight loss over a period of 1 wk or more; or (v) an ulcerated or bleeding tumor).
/> d. The age is recorded as the best estimate of its lifespan.Definitions and calculations
AL: ad libitum
40% dietary restriction (DR): 60% of the volume consumed by ad libitum (AL) fed mice (determined for each strain)
NMR: nuclear magnetic resonance used in whole-body-composition analysis
QMR: Quantitative magnetic resonance (QMR) system measures whole body fat mass, lean tissue mass, free water, and total body water in live mice, without the need for anesthesia or sedation, in less than 1 min.
Data collected by investigator
- body weight
- lean tissue mass
- fat tissue mass
- lifespan: age in days
MPD computed
percent fat
References
Ikeno Y, Hubbard GB, Lee S, Richardson A, Strong R, Diaz V, Nelson JF. Housing density does not influence the longevity effect of calorie restriction. J Gerontol A Biol Sci Med Sci. 2005 Dec;60(12):1510-7.
PubMed 16424282 Liang H, Masoro EJ, Nelson JF, Strong R, McMahan CA, Richardson A. Genetic mouse models of extended lifespan. Exp Gerontol. 2003 Nov-Dec;38(11-12):1353-64.
PubMed 14698816 Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010 Feb;9(1):92-5. Epub 2009 Oct 30.
PubMed 19878144 Miller RA, Harrison DE, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Strong R. An Aging Interventions Testing Program: study design and interim report. Aging Cell. 2007 Aug;6(4):565-75. Epub 2007 Jun 18.
PubMed 17578509 Mobbs CV, Bray GA, Atkinson RL, Bartke A, Finch CE, Maratos-Flier E, Crawley JN, Nelson JF. Neuroendocrine and pharmacological manipulations to assess how caloric restriction increases life span. J Gerontol A Biol Sci Med Sci. 2001 Mar;56 Spec No 1:34-44.
PubMed 12088210 Rikke BA, Liao CY, McQueen MB, Nelson JF, Johnson TE. Genetic dissection of dietary restriction in mice supports the metabolic efficiency model of life extension. Exp Gerontol. 2010 Sep;45(9):691-70Epub 2010 May 7.
PubMed 20452416