Kas1 project protocol
Variations in ventral root axon morphology and locomotor behavior in males of 6 inbred strains of mice (2009)
Kas MJ, de Mooij-van Malsen JWith: JG, Yu KL, Veldman H, Oppelaar H, van den Berg LH, Olivier B
Project protocol — Contents
Workflow and sampling
Equipment
Reagents, supplies, and solutions
Procedures for measuring motor activity and neuron size
Data
References
Workflow
Mice are trained on the balance beam after 15 min of adaptation to testing conditions during the dark phase of the circadian cycle Epon resin embedded ventral roots are sectioned and stained Stained, micrographed sections are analyzed • Home cage monitoring system
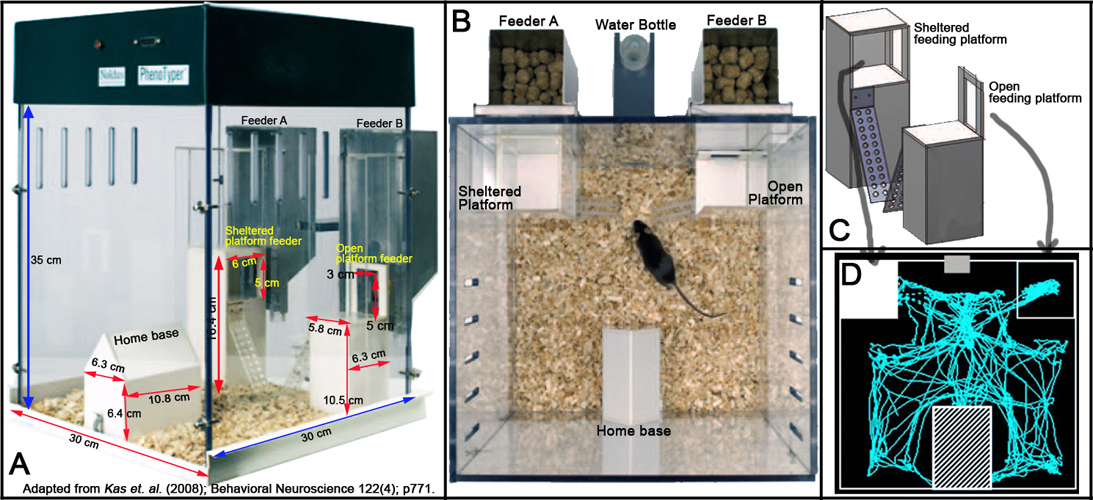
- The home cage measured 30 cm length x 30 cm width x 35 cm height.
- Inside the cage is an enclosed shelter (home base) measuring 10.8 x 6.3 x 6.4 cm, and two different feeding platforms (5.8 x 6.3 x 10.5 cm).
- The outer walls, the shelter home base, and the feeders are all made from Plexiglas (Perspex).
- Within the outer walls of the home cage contained the two feeding platforms.
- In one feeding platform mice are able to eat while exposed to the environment, in the other they are able to eat while sheltered on a feeding platform with a height of 5.9 cm. Both platforms provided ad libitum access to food.
- Behavioral monitoring is accomplished using the PhenoTyper system (Noldus Information Technology, Wageningen, the Netherlands). - The PhenoTyper is a videobased observation system for automated monitoring of rodent behavior.
- Automatic monitoring for consecutive days by video-tracking system is integrated with the ETHOVISION ® (Noldus Information Technology, Wageningen, the Netherlands).
- Within the top of the home cage contained an infrared-sensitive CMOS camera and an array of infrared light-emitting diodes (LEDs) that are connected to a computer running the ETHOVISION 3.0 video-tracking software (see Figure 1 below). Videotracking is performed at a rate of 12.5 samples/s.
Figure 1. Schematic illustration of the Home Cage Monitoring System.
• Balance beam apparatus
- The balance-beam apparatus is composed of (1) a circular platform with 7 cm in diameter and (2) a narrow beam measuring 1.5 cm in width x 27.5 cm in length, (3) which is connected to a goal/destination box (12.7x11.2x10 cm3).
- Lighting is provided by a desk light (illumination level at 400 lux).• Wire grid apparatus
- The wire grid apparatus measured 22 x 28.5 cm2 with a gap width of 1 cm.
- Illumination is provided at a level of 130 lux.
- Motor performance of each mouse is recorded on videotape and analyzed using Observer® (version 3.0, Noldus Information Technology, The Netherlands).• Balance scale
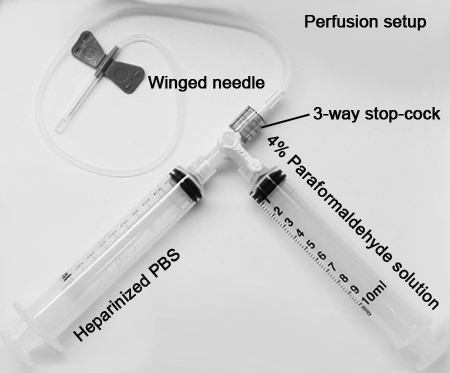
• Perfusion kit (2 x 10 mL syringes, 3-way stop-cock, Butterfly needle)
• Small rodent dissecting/surgical pack (forceps, scissors etc.)
- Pyramitome for making 1 µm-thick sections of neurons (LKB. Ltd, Sweden)
- Dissecting microscope and light microscope with Digital Camera
- ImageJ software (developed by Wayne Rasband, National Institute of Mental Health, Bethesda, MD, USA)
- 70% alcohol
- paper towels
- Pentobarbital sodium (Lundbeck Inc./Ovation Pharmaceuticals Inc., DeerWeld, IL, USA)
- Phosphate-buffered saline (PBS) with heparin
- Needles and syringes
- Freshly prepared 4% paraformaldehyde (PFA; pH 7.30)
- 2% osmium tetroxide
- Serially increasing concentration of acetone
- Epon-acetone
- Epon resin
- Toluidine Blue stain
- Paper towel
Procedures for measuring motor activity and neuron size
I. Measuring activity using automated home cage monitoring system
a. Male 5-7 mo age mice are tested 1 h before the start of the dark phase of the circadian cycle.
b. Mice are weighed and placed individually in a modified home cage environment equipped with automated monitoring system for 73 h without experimenter interference or intervention taking place.
c. Activity in the form of horizontal distance traveled are tracked using the PhenoTyper system. The PhenoTyper is a videobased observation system for automated monitoring of mice behavior. Integrated with the system is ETHOVISION ®, wherein mice are automatically monitored and videotracked for several consecutive days.
d. Videotracking is performed at a rate of 12.5 samples/s. During the 3 consecutive days, consumptive behaviors (drinking and feeding attempt frequency and duration) and behavioral monitoring are registered and performed with infrared sensors in individually housed mice.
e. Distance traveled, entrance frequency and duration spent within the shelter, in addition to the 2 feeding platforms, are also recorded.
f. Data are analyzed for the 1st h of testing to measure the effect of novelty on activity, and for the following days per 24 h.
g. The Plexiglas home cages, shelter, and feeders are thoroughly cleaned after each used and lined with fresh bedding, food, and water (see Figure 2 below).
Figure 2. Physical layout of the modified Home Cage Monitoring System. Panel A shows the lateral view. Panel B shows the dorso-ventral view. Panel C shows the schematic layout of the feeding platforms. Panel D shows a schematic representation of horizontal distance traveled or scanned activity of a mouse.
II. Measuring motor coordination and activity using the balance beam task
a. The balance beam task is performed in a room provided with a desk light with illumination level of 400 lux.
b. The task is accomplished during the dark phase of the circadian cycle.
c. Mice are adapted to the test room 15 min prior to the task.
d. Mice are allowed to train once a day for 4 days, followed by a test day.
e. The maximum trial duration is set to 10 min.
f. Each mouse is placed on a circular platform at the start of each session.
g. The latencies of exploring the circular platform and eventual crossing of the balance beam to reach the goal-box or destination are recorded.
h. Between sessions, the balance beam apparatus is thoroughly cleaned with 70% ethanol.
III. Measuring limb strength and endurance using the inverted wire grid test
a. The inverted wire grid task is performed during the dark phase of the circadian cycle in a room with illumination level of 130 lux.
b. Mice are allowed to train once a day for 4 days, followed by a final test day.
c. During training a mouse is allowed to hang on the inverted grid with its fore-limbs and hind-limbs for 5 min duration.
d. Mice that are able to hold on for 5 min passed and are eligible for the final test.
e. On the test day, the motor performance of each mouse is recorded on videotape.
f. The frequencies of alternating hind limb movement and hopping (synchronous) hind limb movement are analyzed and recorded using Observer® software.
g. In between test sessions, the wire grid is thoroughly cleaned with 70% ethanol.VI. Ventral root collection, processing, and analysis
a. 7-8 month-old male mice that have gone through the battery of behavioral and motor activity testing are anesthetized with pentobarbital intraperitoneally (i.p.).
b. Once proper depth of anesthesia is achieved, the mice are prepared for intracardiac infusion of phosphate-buffered saline (PBS) with heparin:1. One 10 mL syringe is filled with heparinized PBS and attached to one side of a 3-way stop-cock, another syringe filled with freshly prepared 4% paraformaldehyde (PFA; pH 7.30, see perfusion set-up) is attached to the other side.
2. A 23 gauge Butterfly or winged-needle is attached to the 3-way stop-cock. The 3-way stop-cock is turned "ON" to deliver heparinized PBS first. The set up is initially primed to eliminate air bubbles in the line that will potentially block a successful perfusion.
3. The deeply anesthetized mouse is then positioned ventral side up in a dissecting tray-within a well-ventilated hood.
4. Using scissors and forceps, the ventral skin is trimmed from the lower abdomen through the thorax, and mandible.
5. The abdominal wall is then cut and lifted upwards over the thoracic cavity.
6. The diaphragm is incised along both sides of the rib cage-while the abdominal wall tissue is removed. Starting from the xyphoid, the rib cage is cut and folded upwards towards the head to expose the heart.
7. With the heart visibly exposed, a nick into the right ventricular wall is made to allow blood to flow out from the heart.
8. As soon as blood flows out of the right ventricle, the butterfly or winged needle is inserted into the left ventricle of the heart.
9. Using a steady, gentle pressure, between 5 and 10 mL of heparinized PBS is perfused through the mouse. Enough pressure is used to push the blood out of the system, but not so much that the organs are potentially damaged.
10. Successful perfusion is accomplished when blood flow out of the right ventricle become visibly less, and as the PBS replace it, the perfusate become paler concomitant with the organs also becoming lighter in color.
11. Once the mouse is perfused with heparinized PBS, the 3-way stop-cock is turned "ON" to the side of the syringe containing the 4% paraformaldehyde fixative. Careful not to dislodge the winged needle in the left ventricle.
12. Again using steady, gentle pressure, the body is perfused with a similar amount of fixative. If the fixation is successful; the body will begin to twitch and stiffen from the tip of the tail to the nose and all the organs will become even lighter in color.
13. The perfused mice are then post-fixed overnight in 0.4% PFA at 4 °C until dissection.
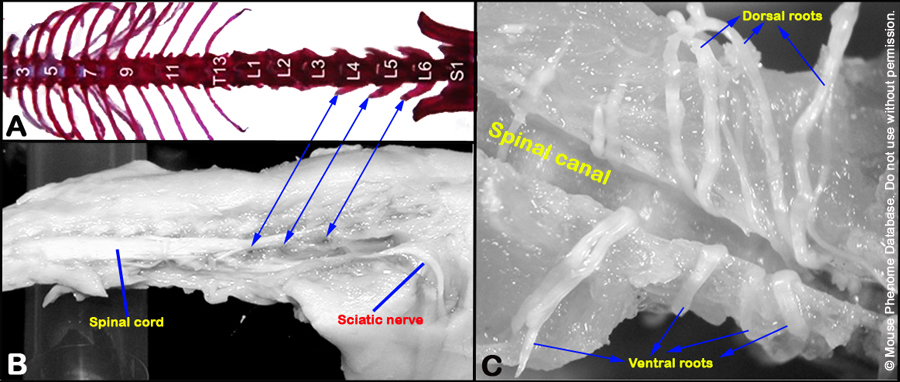
c. The ventral roots (left and right) exiting the spinal cord at the level of L4 lumbar vertebrae are carefully dissected and isolated with the aid of a dissecting microscope.
d. The ventral roots, composed of axons originating from the motor neurons in the L4 spinal cord (see Figure 4 below), are stored in 0.4% PFA, and then post-fixed in 2% osmium tetroxide, dehydrated in serially increasing concentration of acetone and in Epon-acetone mixture overnight.
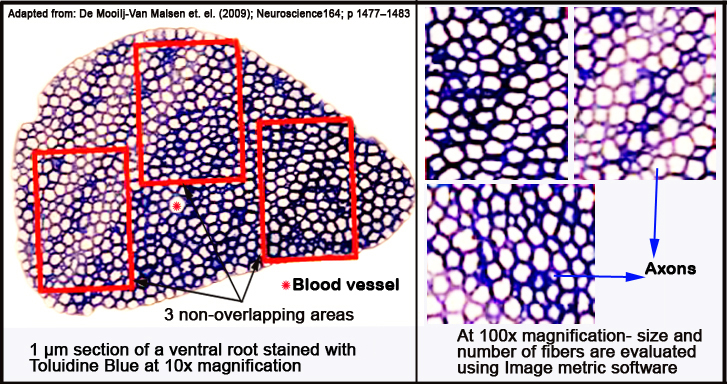
e. Finally the ventral roots are embedded in Epon resin, sectioned into 1 µm-thick slices with a pyramitome (microtome) and stained with Toluidine Blue for light microscopy.
f. Per ventral root, three to four sections are examined.
g. Total root size is examined with a 10x magnification; close-up analysis is performed by taking three randomly chosen, non-overlapping fields within the root (see Figure 3 below) with a 100x magnification.
h. Micrographs are obtained with a digital camera, while the size and number of myelinated fibers are analyzed with ImageJ.
Figure 3. Schematic layout of micrographed ventral root areas analyzed with ImageJ morphometric software.
Data collected by investigator
- Home cage plus monitoring system: total distance traveled, 1st-hr in novel home cage and total distance traveled on the 3rd (24 h) day in home cage.
- Balance beam: latency to reach box via a narrow beam.
- Inverted wire grid test: latency to fall on inverted grid and number of alternating hindlimb movement on inverted grid after 5 min.
- Ventral root morphometry: area of ventral root (µm2), number of axons per ventral root size, area of median axon (µm2), number of 0-50 µm2 axons, number of 50-100 µm2 axons, number of 100-150 µm2 axons, number of 150-200 µm2 axons.
Definitions and calculations
ventral root: contains axons originating from the motor neurons in the spinal cord.
axon: is a long, slender projection of a nerve cell, or neuron, that is usually myelinated and conducts electrical impulses away from the neuron's cell body or soma to muscles it innervates.
Figure 4. Panels A and B show the location of the L4 ventral root. Panel C shows isolated ventral and dorsal roots.
References
de Mooij-van Malsen AJ, Olivier B, Kas MJ. Behavioural genetics in mood and anxiety: a next step in finding novel pharmacological targets. Eur J Pharmacol. 2008 May 13;585(2-3):436-40. Epub 2008 Mar 18.
PubMed 18420190 de Mooij-van Malsen JG, van Lith HA, Oppelaar H, Olivier B, Kas MJ. Evidence for epigenetic interactions for loci on mouse chromosome 1 regulating open field activity. Behav Genet. 2009 Mar;39(2):176-82. Epub 2008 Dec 2.
PubMed 19048365 de Visser L, van den Bos R, Kuurman WW, Kas MJ, Spruijt BM. Novel approach to the behavioural characterization of inbred mice: automated home cage observations. Genes Brain Behav. 2006 Aug;5(6):458-66.
PubMed 16923150 Gelegen C, Pjetri E, Campbell IC, Collier DA, Oppelaar H, Kas MJ. Chromosomal mapping of excessive physical activity in mice in response to a restricted feeding schedule. Eur Neuropsychopharmacol. 2010 May;20(5):317-26. Epub 2009 Nov 7.
PubMed 19896807 Kas MJ, de Mooij-van Malsen AJ, Olivier B, Spruijt BM, van Ree JM. Differential genetic regulation of motor activity and anxiety-related behaviors in mice using an automated home cage task. Behav Neurosci. 2008 Aug;122(4):769-76.
PubMed 18729629 Kas MJ, Fernandes C, Schalkwyk LC, Collier DA. Genetics of behavioural domains across the neuropsychiatric spectrum; of mice and men. Mol Psychiatry. 2007 Apr;12(4):324-30. Epub 2006 Nov 21.
PubMed 17389901 Kas MJ, Van Ree JM. Dissecting complex behaviours in the post-genomic era. Trends Neurosci. 2004 Jul;27(7):366-9.
PubMed 15219734 Noldus LP, Spink AJ, Tegelenbosch RA. EthoVision: a versatile video tracking system for automation of behavioral experiments. Behav Res Methods Instrum Comput. 2001 Aug;33(3):398-414.
PubMed 11591072