Donahue1 project protocol
Bone mineral density, body composition, and craniofacial characterization in 30 inbred strains of mice (2002)
Donahue LWith: DeMombro V, Maynard J, Bauschatz J, Hurd J, Marden C, Curtain M
Project protocol — Contents
Workflow and sampling
Workflow and sampling
Equipment
Procedures
Data
Definitions and calculations
References
Workflow
Whole body weight measurement bw Mice are euthanized by decapitation Whole blood is collected and process for serum hormone assay serum thyroxine (T4), insulin-like growth factor 1 (IGF-1), osteocalcin (OC) Mice are scanned for body composition BMD, BMC, fat and lean mass Head is dissected and processed for skull metrics length, height and width of the skull and jaws; nose length and right-left medial canthus (eyes) distance
- Ohaus Portable Navigator Series Electronic Toploading Balance (Model NV-210) for measuring body weights
- Digital hand calipers (Stoelting, Wood Dale, IL, USA)
- Heavy-duty sharp decapitating scissors
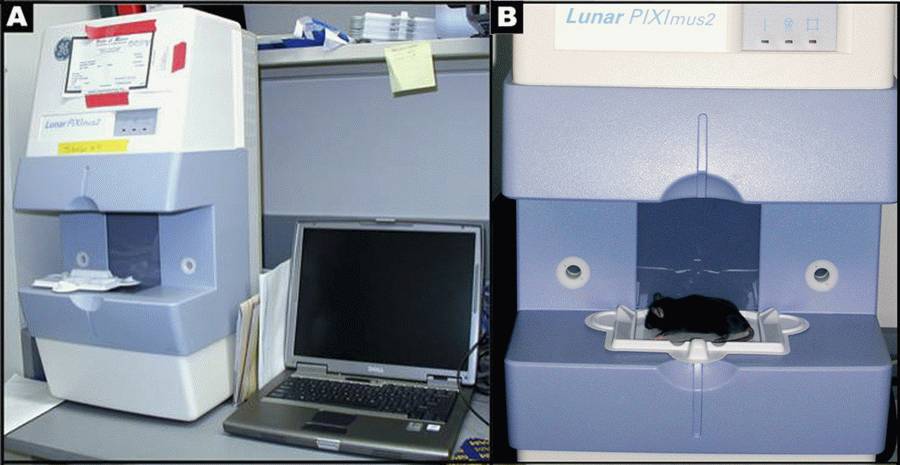
- DXA scanning by PIXImus: The PIXImus small animal DXA system (PIXImus™, Fitchburg, WI) is used to assess whole body areal (a) BMD and body composition at 16 weeks of age. This methodology has been validated in small animals (see Nagy 2000).
- 1.5 mL microcentrifuge tubes for blood collection
- Disposable specimen trays with sticky immobilizing tape: Lunar PIXImus Corporation Headquarters, 726 Heartland Trail, Madison, WI 53717
Mouse densitometer dual energy X-ray absorptiometry (PIXImus small animal) DXA system (GE-Lunar, Madison, WI): The PIXImus mouse densitometer has been reconfigured with lower x-ray energy than in human DXA machines in order to achieve optimal contrast in small specimens. The Lunar PIXImus for rodents is a fully integrated densitometer designed for the estimation of bone mineral density (BMD) and body composition. The resolution of the PIXImus is 0.18 x 0.18 mm pixels with a usable scanning area of 80 x 65 mm, allowing for measurement of a single mouse or collections of isolated specimens. The PIXImus has been calibrated with a phantom utilizing known values, and a QA is performed daily with this same phantom. The precision for BMD is less than 1% coefficients of variations (CV) for whole body, approximately 1.5% CV for specialized regions. Correlation with pQCT values for 614 isolated spinal vertebrae is significant (p<0.001; r=.704). Assessment of accuracy for the PIXImus is done with a set of hydroxyapatite standards (0-2,000 mg), yielding a correlation of 0.999 between standards and PIXImus measurement of mineral. Full body scans and X-ray absorptiometry data are processed with manufacturer supplied software (Lunar PIXImus 2,vers. 2.1). For additional information: Lunar PIXImus
Figure 1 A: Lunar PIXImus2 densitometer with integrated PC computer. Figure1 B: Close-up detail of the Lunar PIXImus2 densitometer with specimen tray.• For Synchron CX5 parts and clinical chemistry reagents
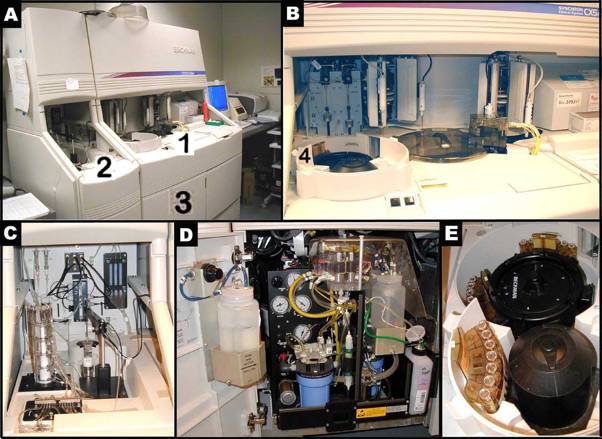
Figure 2 A-E illustrate the Beckman Synchron CX5 Delta. Figure 2 B shows a closer look of area 1 in Figure 2A. Figure 2 C presents a closer look of area 2 in Figure 2A. Figure 2 D depicts a closer view of area 3, where ancillary reagents are refilled in Figure 2A. Figure 2 E reveals the content of area 4, where samples are set up in trays for an automated run in Figure 2B.
- IGF-1 (Direct, IGFBP-blocked) RIA kit, catalog no. 22-IGF-R20, American Laboratory Products Company (ALPCO, Windham, NH)
- 1% KOH for macerating and clearing tissues around bones
- Alizarin red dye for staining skeletal preparations
- Glycerin for storing skeletal preparations
I. Collecting image scans
PIXImus scanning of mice for BMC and % fat is both accurate and precise although body size must be considered when comparing inbred strains. Full body scans are obtained and X-ray absorptiometry data gathered and processed with manufacturer supplied software (version 1.43.036.008).
a. The PIXImus densitometer apparatus is first calibrated with a "phantom mouse" according to manufacturer's protocol.
b. To obtain the PIXImus scans, 16-wk old mice are weighed and body weight recorded, and then are sacrificed by decapitation. (Blood samples are collected for serum hormone assay; see below.)
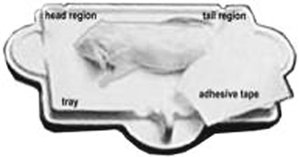
c. Decapitated mice are positioned dorso-ventral with the tail positioned away or alongside from the body, the front legs extended to the side, and the neck and spine are gently straightened.
d. Then each mouse is placed on the specimen sticky tray (body must be within blue line on the tray) under the PIXImus beam path (see Figure 1B above). The tail is placed alongside the body, the front legs are extended to the side, and the neck and spine are gently straightened.
e. Trays are positioned such that the area of the head is always oriented toward the left from the investigator's point of view, and the mice are position dorso-ventral- in order to scan the entire body and tail. The X-ray process to obtain a single full scan is approximately 5 minutes; data can be manipulated subsequently to obtain specific regions of interest (ROI's).
f. After the whole body (excluding the head) is scanned, the isolated head alone is also scanned.
g. Disposable plastic trays, with sticky tape for immobilizing mice, can be saved and re-used after a thorough cleaning and disinfections.

II. Measurement acquisition and image scan analysis
Based on PIXImus validation studies (see Nagy 2000), DXA-estimated measurements of fat tissue correlate well with measurements obtained from chemical extraction. This is made possible by developing software versions with equations that adequately correct raw DXA measurements.
a. Following the completion of an image scan the DXA system automatically implements specialized software to identify bone tissue from either fat tissue or from lean tissue based on the resulting X-ray densities at two distinct energy levels (Pietrobelli, 1996).
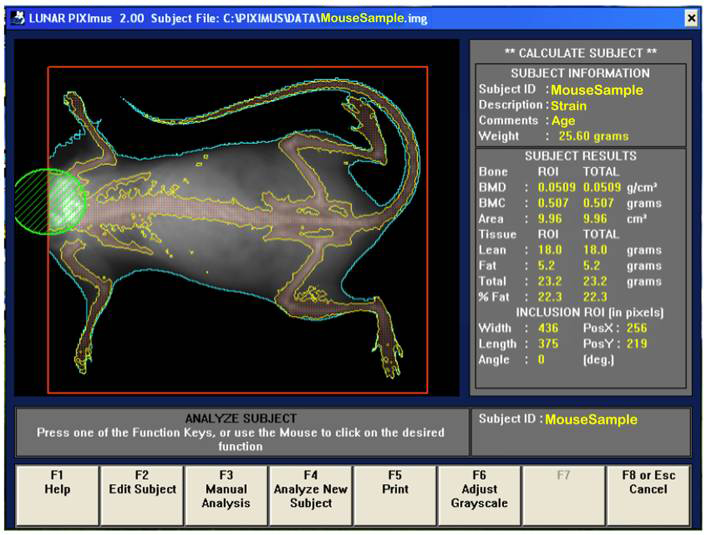
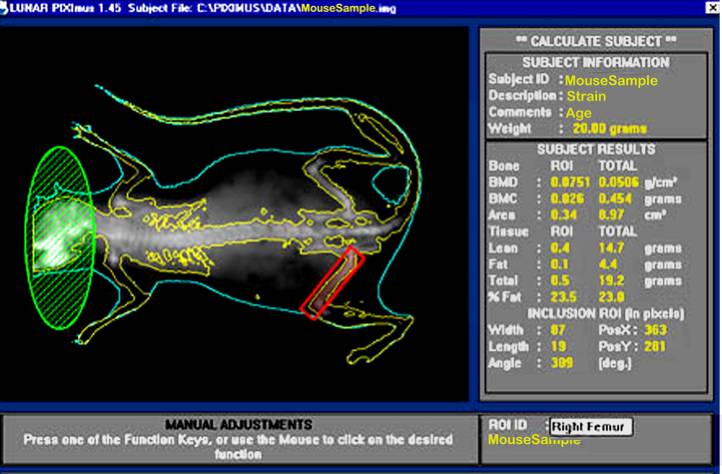
b. Visually, following the completion of a scan, the mouse sample is then outlined with red and green colored circle and square to define specific regions of interests (ROIs).
d. By using the screen interactive display, F3 is first clicked to prompt measurement adjustments, and then clicked again for the second time to adjust ROI (region of interest).
e. The area to be analyzed is defined (red box), and areas to be excluded from the calculations are defined (green). The arrow keys are used to adjust to the desired size, in addition to holding the control key down to enlarge or elongate the circle or square areas.
f. Once the desired ROI is achieved, the Enter key is clicked and resulting data measurement is displayed. By pressing F5 a hard copy of the image and the scan analysis result is printed.
g. To prompt the computer to finish the session, F8 or Esc key is clicked once, and then clicked again to return to the main menu screen where the next subject to be tested begin.
h. Acquired data is saved on the hard drive and on a zip or CD disk for later archiving.III. Serum hormone measurements
Serum thyroxine (T4), osteocalcin (OC), and Insulin-like Growth Factor-1 (IGF-1) were chosen as factors likely to be important in regulating body composition or BMD components that are the focus of our investigations.a. Blood samples are obtained from decapitated mice devoid of effects from excess handling stress or serum dilution with euthanasia injection vehicle.
b. Whole blood samples are collected in 1.5 mL centrifuge tubes and allowed to clot on ice for 3-4 h.
c. The clotted blood samples are then centrifuged at 10,000 RPM for 5 min.
d. The top serum layer is carefully harvested and stored at –20°C for subsequent hormone assays.
e. Serum thyroxin (T4) is measured in duplicate 9 µL volumes at The Jackson Laboratory using the Beckman CX5 Clinical Chemistry Analyzer, with data expressed as ng/mL.
d. Serum osteocalcin (OC) is measured in duplicate 25 µL volumes using the mouse specific assay (Gundberg, 1992) developed by Dr. Caren Gundberg in her laboratory (Yale University, New Haven, CT), with data expressed as ng/mL.
f. Serum IGF-1 levels are measured in duplicate 25 µL volumes using kits obtained from ALPCO. (When using the radioimmunoassay technique IGF-1 is dissociated from the binding proteins (IGFBP's) by dilution in an acidic solution. This acidic solution is then neutralized with the addition of a buffer solution, containing antibody against IGF-1 plus an excess IGF-2. Because IGF-2 is presented in excess, it (competitively) occupies the IGF-binding sites and the displaced IGF-1 is measured through the addition of an I125 tracer. Separation of the bound I125 (or IGF-1 bound to IGFBP’s) and free I125 tracer (or competitively displaced IGF-1) is carried out by the addition of a second antibody (see schematic figure).
g. Values are normalized to a standard C57BL/6J pooled serum sample that is run on each assay to avoid interassay variability. This is reflected in the data as "adjusted IGF-1" values.IV. Skull or skeleton preparations for measuring bone dimensions
a. The skin and subcutaneous tissue around the head and/or body are removed in order to isolate the skull and skeleton.
b. In many cases whole skeletons of mice are cleared and attached tissues are incompletely macerated with 1% potassium hydroxide (1% KOH).
c. Cleared and tissue-free skeletons and cartilages are stained with alizarin red dye.
d. Stained skeletons are then stored in undiluted glycerin (Green, 1952) for subsequent visual evaluation and/or caliper measurement.
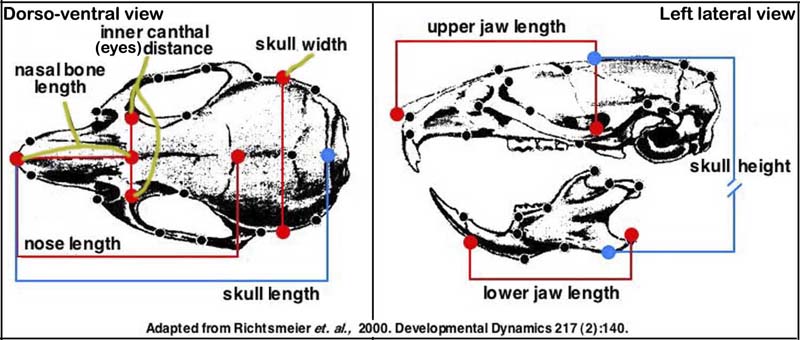
e. Seven measurements taken with hand held digital calipers are used routinely to define skull morphology (see image below).
f. Malformations found during visual evaluation and caliper measurements can indicate that the craniofacial (skull) phenotype is part of a greater syndrome.
g. For additional information see the Craniofacial Mutant Resource at TJL (Bauschatz et al., 2003).
Note from submitting investigator: These measures have a high degree of accuracy and precision in our hands and are able to discriminate differences between mutant and control skull characteristics. Our linear measures have been added to the illustration below which was taken from a publication by Dr. Joan Richtsmeier characterizing craniofacial differences in mouse models of Down Syndrome using three dimensional anatomical landmarks (Richtsmeier, 2000).
Safety
For safety, gloves must be worn and radiation safety guidelines are strictly adhered to, such that technicians must be 6 feet away from the PIXImus machine during scanning.
ROI = Region of interest
Bone area measurement is generated by outlining or specifying the limits or dimensions of the entire skeletal bone regions of the body (limbs, neck, spine, and tail), excluding the head, as regions of interest (ROI) following a full body X-ray scan.
Bone mineral content (BMC) is generated from PIXImus density scans which are assessed for accuracy using a set of 0.0 mg to 2,000 mg of hydroxyapatite standards. According to the DXA system, bone mineral content (measured as the attenuation of the X-ray by the bones being scanned) is divided by the area (also measured by the machine) of the site being scanned to obtain bone mineral density (BMD):
BMC = Bone mineral content [g]
BMD = Bone mineral density = BMC ÷ Area [g/cm2]
Fat tissue mass = all tissues with low density (x-ray scan) [g]
% Fat = (Fat tissue mass ÷ Total body tissue mass ) x 100
Data collected by investigator
Body weight, body composition (fat and lean mass, total tissue mass, BMD, BMC), facial dimensions (skull and jaw length, height, and width; nose length, and eye distance), serum hormone levels (thyroxine (T4), insulin growth factor-1 (IGF-1), osteocalcin (OC)).
References
Bauschatz JD, Curtain MM, Davisson MT, Lane PW, Donahue LR. In collaboration: the Jackson Laboratory Craniofacial Resource. Crit Rev Eukaryot Gene Expr. 2003;13(2-4):107-8.
PubMed 14696959 Green, M.C. A rapid method for clearing and staining specimens for the demonstration of bone. The Ohio Journal of Science 52(1):31-33. January 1952.
Gundberg CM, Clough ME, Carpenter TO. Development and validation of a radioimmunoassay for mouse osteocalcin: paradoxical response in the Hyp mouse. Endocrinology. 1992 Apr;130(4):1909-15.
PubMed 1547718 Nagy TR, Clair AL. Precision and accuracy of dual-energy X-ray absorptiometry for determining in vivo body composition of mice. Obes Res. 2000 Aug;8(5):392-8.
PubMed 10968731 Pietrobelli A, Formica C, Wang Z, Heymsfield SB. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol. 1996 Dec;271(6 Pt 1):E941-51.
PubMed 8997211 Richtsmeier JT, Baxter LL, Reeves RH. Parallels of craniofacial maldevelopment in Down syndrome and Ts65Dn mice. Dev Dyn. 2000 Feb;217(2):137-45.
PubMed 10706138