Shockley1 project protocol
Diet effects on blood chemistry and lipids in 10 inbred strains of mice (2008)
Shockley K, Paigen B, Churchill GAWith: Witmer D, Burgess-Herbert SL
Project protocol — Contents
Workflow and sampling
Equipment
Reagents, supplies, and solutions
Procedure: Blood chemistry and electrolyte measurement using Beckman CX5 Synchron Delta Chemistry Autoanalyzer
Data
ReferencesAcclimation to test conditions
In general all mice are housed singly before they are tested.
- Refrigerated tabletop micro-centrifuge: Eppendorf Centrifuge, Model # 5415C
- Repeat pipettes and regular Pipetman: 250 µL and 100 µL, respectively
- Automated blood chemistry analyzer: Synchron CX5 Delta (Beckman Coulter, Inc., Fullerton, CA)
- A dedicated DOS-based desktop computer controls the programming of this analyzer
- A dedicated printer prints the results as they are measured, and an electronic file is simultaneously transferred to a second, Windows-based computer, which stores the data files
- Perfusion kit: venous catheters or large needles and 5 mL syringes
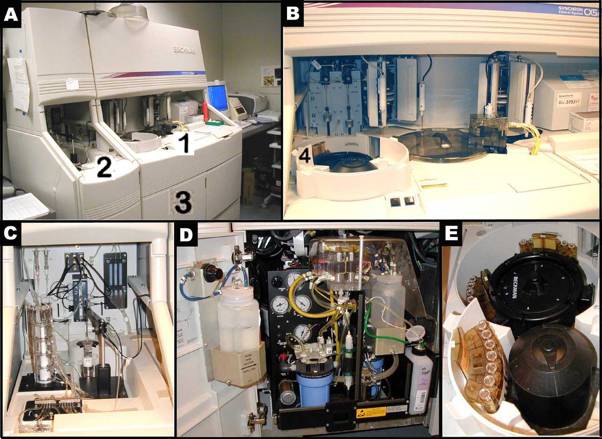
Panels A-E illustrate the Beckman Synchron CX5 Delta. Panel B shows a closer look of area 1 in Figure A. Figure C presents a closer look of area 2 in Panel A. Panel D depicts a closer view of area 3, where ancillary reagents are refilled in Panel A. Panel E reveals the content of area 4, where samples are set up in trays for an automated run in Panel B.
- Micro-hematocrit tubes (75 µL capacity) coated with Heparin and equipped with a rubber bulb expunger
- Heparin anticoagulant (Sigma (Sodium Salt) 50,000 U Cat. #H-3393)
->Heparin 1000 U/mL: 50 mL of distilled autoclaved/sterile water + 50,000 U Heparin Stock Solution- Sterile physiological (0.9%) saline solution
Expendables:
(1) 1.5 µL Eppendorf tube,
(2) 0.5 mL Beckman Coulter Microtube Tubecup ("sector cup")
(3) 100 µL and 250 µL pipette tips
(4) 1 cc syringes with needlesReagents: The Chemistry Analyzer (or "CX5") uses Beckman Coulter three-compartment reagent cartridges for HDL, CHOL, TG, and GLU. Each cartridge contains enough reagents for 300 tests (approximately 104 mL). In addition, in order to run the HDL Cholesterol test, HDL Cholesterol Separation Reagent (15 µL per sample) is needed. The bottle from Beckman Coulter contains a volume of 34 mL. If the dilution of serum samples becomes necessary due to low serum volume, use 0.9% saline solution for the dilution.
Reagent: Reorder No.
Cholesterol (CHOL) Reagent 467825
Glucose (GLU) Reagent 442640
Triglycerides (TG) Reagent 445850Calibration Reagents: The two calibration reagents are "Synchron Systems HDL Cholesterol Calibrator" (for HDL only), and "Synchron Systems Multi Calibrator" (for CHOL, GLU, and TG).
Controls: The controls for HDLc are Beckman Coulter Vigil Lipid Control 1 and Beckman Coulter Vigil Lipid Control 2. The controls for CHOL, GLU and TG are Synchron Control Comprehensive Chemistry Control Serum Level 1, Level 2 and Level 3.
Procedure: Blood serum chemistry and electrolyte measurement using Beckman CX5 Synchron Delta Chemistry Autoanalyzer
I. Pre-test feeding regimen
a. All mice received standard diet of 6% fat until 6-wks of age.
b. At 6-wks of age, the mice are divided into two dietary groups with 5 males and 5 females per strain per group.
c. The 1st group is given the standard low-fat diet, and the second group is given an atherogenic high-fat diet (30% Kcal from dairy fat) containing by weight 1% cholesterol and 0.5% cholic acid to increase cholesterol uptake.
d. The 2 dietary groups are then kept on the feeding regimen for 4 consecutive weeks.
e. At 10–13 wk old and/or after 4 wks on the dietary regimen, the mice are single-housed for 3 days before blood sampling (see below).
f. On the day of the blood collection, the mice are placed in clean box cages without food from 6 a.m. to 11 a.m.
g. Following blood collection the mice are subsequently euthanized by cervical dislocation between 11 a.m. to noon (to minimize circadian variation in mRNA expression), and then perfused with saline before the liver is dissected and the median lobe is harvested and cut into 1 cm2 sections to be placed in RNAlater® at room temperature for 24 hrs, then stored at -80°C until RNA extraction for microarray is done.Investigator's notes: "Due to difficulties associated with obtaining blood samples from CAST/EiJ and PERA/Ei mice, ages at bleeding and death ranged from 20 to 55 wks of age. We removed these strains from our primary analysis to eliminate unwanted variability from this age difference. However, the data are available via the CGD website..."
II. Blood serum collection
a. At 10-13 wks of age and after a 5-hr fast from 6:00-11:00 a.m., each mouse is weighed and blood sample obtained between 11:00 a.m. to noon to minimize sampling variability.
b. Approximately 200 µL of blood is obtained from each mouse via retro-orbital bleed using heparin-coated Hematocrit tubes. Remaining blood in the Hematocrit tube is flushed out using a rubber squeeze bulb assembly.
c. Blood samples are collected into pre-labeled 1.5 mL Eppendorf tubes and momentarily stored in ice until all the samples are ready to be centrifuged.
d. Blood samples are then centrifuged for 5 min at 14,000 RPM using a refrigerated table-top micro-centrifuge to separate the serum.
e. The top serum layer is pipetted into pre-labeled 0.5 mL Eppendorf tubes and frozen until ready to be assayed; remaining packed blood cell layer is discarded.
f. Previously frozen serum samples are defrosted at room temperature for about 30 min before measurements are done.NOTES: Air bubbles are avoided and eliminated during sample loading in sector cups as they interfere with the colorimetric assay.
III. Using Beckman CX5 Synchron system to measure serum glucose and lipids chemistries

Panel F shows 2 empty sector cups. Panels G shows sector cups with/out hemolyzed sample, and Panel H shows sector cups without and with (red arrow) air bubbles. Panels I and J show consecutive sector trays identified with bar codes and seven sector cups contained within each tray.
a. In preparation for the auto-analyzer, bar-coded sectors with cups in place are loaded with 85 µL of completely thawed serum (enough for the direct measurement of CHOL, GLU, and TG).
b. Up to five sectors are manually placed on the carousel to be run. Since each sector is bar-coded, the Beckman automatically detects a sector that has been run; regardless, finished sectors are removed immediately as soon as they come up.
c. The Beckman CX5 is operated according to manufacturer's instructions in the measurement of serum HDL, CHOL, GLU, and TG, which are run together.
d. -Function key F1 is used to deploy "Sample Program", Sample type "1" is for serum.
-Function key F2 is used to deploy "Program Batch/Sector(s)" and "sector(s) to program:" (i.e. sectors 1-5 is programmed). When "Batch" mode activated, 7 cups possible. "Number of cups in batch:" message is displayed, the total cups for this batch is then (7 x 5) = 35 cups (the maximum number of cups that can be run in a batch is 98). To program remaining sectors, F2 Program Batch is deployed again.
-Panel "12" is preprogrammed for CHOL/GLU/TG and "HDLD" is manually "Selected" from the screen menu. Once the correct chemistries are selected, they are "ENTERed" to bring about "SAMPLE TYPE", wherein "1" is given to denote serum (not plasma).
-Function key F8 is used to set up the programmed batches and to advance to the next cup/sample (from cup #1 up to cup #7) in a given sector. By selecting F8 ID numbers can be recorded and reviewed against an Excel reference sheet.Notes: Since ID sample numbers or field identifiers are un-editable once Beckman CX5 is in operation, relevant information, including sample type (i.e. plasma vs serum), dilution factor, and other information must be precisely entered.
• As soon as six sectors are programmed, the <prev screen> is activated first, and then the Master Screen, and then last START (green) button. The Beckman automatically starts sampling the first five programmed sectors. Additional sectors can be programmed while the Beckman is operating.
• In the event that the Beckman alarm is activated because of reagent volume getting low, <prev screen> is activated to turn off the alarm and make the necessary notations for the record. The next available reagent cartridge is automatically installed.
• When all the data is safely recorded, Function key F5 is used to clear all the information regarding a sector after ENTERing the number of the sector to be deleted.e. Once a sector is finished running, the results are automatically printed, removed from the printer, and then labeled accordingly. Otherwise, the printed paper is checked and guarded from rolling back into the printer and disrupting the data recording.
f. Used Eppendorf tubes, pipette tips, sector cups, and reagent cartridges are discarded into biohazard waste containers, and any spilled liquids are cleaned.Definitions & formulas
The average of the diluents analyzer value is subtracted from the analyzer value multiplied by two (dilution factor) to obtain the HDL (indirect) values.
HDL (indirect) values = (sample analyzer value x 2) - average of diluents analyzer value
Data collected by investigator
Cohorts:
control diet
high-fat dietdifference*
- blood chemistry
- calcium (Ca)
- glutamate dehydrogenase (GLDH)
- glucose (GLU)
- blood urea nitrogen (BUN)
- blood lipids
- HDL cholesterol (HDL)
- total cholesterol (CHOL)
- nonesterified fatty acid (NEFA)
- triglycerides (TG)
- body weight
- endocrine hormones
- thyroxine (T4)
*MPD Note: "Difference" vectors were computed by MPD from the Shockley1 strain means (high fat diet over control), and are available for download in a separate supplementary file.