Mogil1 project protocol
Investigation of basal nociception in males of 12 inbred strains of mice (2002)
Mogil JS, Devor M, Lariviere WRWith: Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Laughlin TM, Kokayeff A, West EE, Adhikari SM, Wan Y
Project protocol — Contents
Workflow and sampling
Equipment
Reagents, supplies, and solutions
Procedures for conducting a battery of nociceptive tests
Data
Definitions
References
Workflow
The nociceptive assays are not necessarily conducted in sequence unless otherwise described in the individual procedures.
Abdominal constriction Test 0.6% acetic acid i.p. number of nociceptive "writhes" Abdominal constriction Test magnesium sulfate i.p. number of nociceptive "writhes" Autotomy/denervation test sciatic and saphenous denervation autotomy score (1-11) Formalin test 5% formalin s.q. duration of nociceptive licking/biting of treated hindpaw Formalin test 5% formalin s.q. duration of nociceptive licking/biting of treated hindpaw Hargreaves' test; 6x trials high intensity light (heat) to the plantar surface latency of hindpaw withdrawal Hot Plate test hot metal surface latency of nociceptive hindpaw licking, shaking or fluttering Thermal hypersensitivity following peripheral nerve injury L5 spinal nerve injury and high intensity light (heat) to the affected plantar surface percent thermal hypersensitivity of the surgically treated hindpaw relative to the un-treated hindpaw Mechanical Hypersensitivity following peripheral nerve injury L5 spinal nerve injury and mechanical stimulation (prick) with von Frey monofilament percent mechanical hypersensitivity of the surgically treated hindpaw relative to the un-treated hindpaw von Frey monofilament test single steady prick with calibrated von Frey monofilament mechanical threshold/ stimulation for foot withdrawal Intrathecal dynorphin-induced hypersensitivity and von Frey test intrathecal (i.t.) dynorphin-induced hypersensitivity and allodynia with von Frey monofilament mechanical hypersensitivity to von Frey monofilament Thermal hyperalgesia - carrageenan test 2% carrageenan s.q. and high intensity light (heat) to the injected plantar surface percent thermal hypersensitivity of the treated hindpaw relative to the un-treated hindpaw Tail Withdrawal Test; 3x trials 49°C heat stimulation of the tail latency to withdraw tail from 49°C heat stimulation Tail Withdrawal Test; 3x trials 47.5°C heat stimulation of the tail latency to withdraw tail from 47.5°C heat stimulation Tail Withdrawal Test; 3x trials -15°C cold stimulation of the tail latency to withdraw tail from -15°C heat stimulation Bee venom test bee venom s.q. duration of bee venom-induced licking of injected hindpaw Hargreaves' test following bee venom test; 6 trials bee venom s.q. and high intensity light (heat) ipsilateral to the injected plantar surface latency of thermal hypersensitivity following heat stimulation of ipsilateral injected hindpaw Hargreaves' test following bee venom test; 6 trials bee venom s.q. and high intensity light (heat) contralateral to the injected plantar surface latency of thermal hypersensitivity following heat stimulation of contralateral injected hindpaw Capsaicin test capsaicin (2.5 µg/20 µL 2% DMSO) s.q. duration of capsaicin-induced licking of injected hindpaw Tail-withdrawal test after capsaicin test; 2 trials/session capsaicin (2.5 µg/5 µL 2% DMSO) s.q. (mid-tail) and 47±.2°C heat stimulation of the tail latency of tail withdrawal following capsaicin-induced hypersensitivity Tail-clip test alligator clip exerting 500xg of force applied to the tail latency of nociception to mechanical-induced pain Tail-flick test; 3x trials insertion of 2 acupuncture needles in the hind leg and focused light (heat) applied to mid-tail latency of tail-flick to thermal-induced nociception
- Stopwatch
- Plexiglas cylinders (30 cm high; 30 cm diameter)
- Plexiglas chambers (12.5 x 12.5 x 12.5 cm) atop a 3/16-inch glass floor
- High-intensity projector lamp beam (setting = 3; approximately 45 W, IITC Model 336)
- Metal surface heated to 53.0 ± 0.2 degrees C enclosed within a 14 x 14 cm Plexiglas enclosure (IITC Model PE34M-HC)
- Water circulator thermostatically maintained at 49.0 ± 0.2 degrees C (VWR Model 1110 Immersion Circulator).
- Thermostatically controlled immersion cooling device (model IBC-4; Neslab Instruments, Portsmouth, NH)
- Circulating water bath maintained at 47 ± 0.2°C (Isotemp IC2100 Circulator, Fisher Scientific)
- Transparent plastic box (4 x 4 x 7 cm) with a metal mesh floor
- von Frey monofilament (bending forces: 0.3, 0.7, 1.6, 4.0, 9.8, 22.0, and 53.9 mN; applied using a single, steady >1-sec application)
- Transparent Plexiglas cylinders (15 cm diameter x 46 cm high) on a glass floor
- Well-ventilated observation chambers (8.5 x 5 x 5.5 cm3) with transparent outer walls and ceiling, a 3/16th-inch-thick glass floor, and opaque inner walls that visually isolated each mouse from the others
- precision calipers
- alligator clip with rubber cuffs around the teeth of each jaw (exerting 500 x g of force)
- customized cloth/ cardboard holder-mouse restrainer
- normal (physiological) saline = 0.9% saline v/v, autoclaved-sterilized
- silk sutures/threads (5-0, 6-0)
- surgical blades
- 0.6% glacial acetic acid
- 37% formaldehyde stock solution diluted with normal saline to 5% formalin working solution
- 120 mg/kg magnesium sulfate
- Pentobarbital anesthetic (50 mg/kg, i.p.)
- Carrageenan (2%; 20 mg/mL; Sigma)
- 27, 30-gauge needles
- 50 µL micro syringe
- 2.5 µg capsaicin dissolved in 20 µL of vehicle (2% dimethyl sulfoxide (DMSO) in normal saline)
- 3 nmol dynorphin (Tocris Inc.) dissolved in 5 µL of normal saline
- acupuncture needles
- Michel surgical suture clips
- Post-operative antibiotics, systemic and topical preparations
- Gaseous anesthesia with halothane (3.5% for induction and 1.7% for maintenance) in O2 gas
- 50 µg honey bee venom (lyophilized whole venom of Apis mellifera; #V3375, Sigma) dissolved in 25 µL of normal saline
- 3 nmol dynorphin (Tocris Inc.) dissolved in 5 µL of normal saline, dose known to produce allodynia in mice when administered intrathecally
- acupuncture needles (0.15 mm diameter)
- adhesive tape
Acclimation to test conditions
Naïve mice are individually removed from their home cage and acclimated for 1 hr to the testing room, and habituated from 10 min to 2 hr to individual testing chamber.
Procedures for conducting a battery of nociceptive tests
Each of the following assays was performed on a set of mice, e.g., each mouse was used only in a single assay.
I- Abdominal Constriction Test (AC_AA, AC_MS)
a. After acclimating to the testing room for 1 hr, naïve mice are individually habituated for 10 min in Plexiglas observation cylinders (27 x 17 x 12 mm).
b. Then, 0.6% glacial acetic acid or 120 mg/kg magnesium sulfate is injected intraperitoneally (i.p.).
c. Mice are then returned to the cylinders, and behavioral observations are conducted immediately.
d. The total number of "writhes" (lengthwise stretches of the torso with a concomitant concave arching of the back) in 20 min (acetic acid) or 5 min (magnesium sulfate) is counted.II- Autotomy (Denervation) (AUT)
a. Following pentobarbital anesthetic induction, equal number of mice are operated on the right as well as the left hindleg to expose the sciatic nerve at mid-thigh.
b. With good visibility, the sciatic nerve is tightly ligated with 5-0 silk, and transected 0.5-1.0 mm distal to the ligature using a sharp blade.
c. About 5 mm of the distal nerve stump is further excised to suppress regeneration.
d. In the same leg, the saphenous nerve is exposed near the knee, and likewise ligated and cut.
e. Post-operatively, surgical incisions are closed in layers using silk sutures and Michel clips, and the mice are administered topical as well as systemic antibiotics.
f. The mice are then allowed to complete recovery form anesthesia before they are returned to the vivarium.
g. Complete denervation of the hindlimb is verified 1-7 days post-operatively by the absence of a flexion response to pinching the medial and lateral toes of the paw.
h. The extent of autotomy is rated weekly using the scale of Wall et al. (1979).
i. Briefly, one point is given for the removal of one or more toe nails, and an additional point given for injury to each half phalange for a total maximum possible score of 11.
j. Autotomy scores at the 35th post-operative day are recorded.II- Formalin Test (F_early, F_late)
a. Naïve mice are individually removed from their home cage and habituated for 30 min in Plexiglas cylinders (30 cm high; 30 cm diameter) atop a glass floor.
b. Then, 25 µL of 5% formalin is injected subcutaneously (S.Q.) into the plantar surface of the right hindpaw using a 50 µL microsyringe with a 30-gauge needle.
c. Mice are then returned to the cylinders, and behavioral observations are conducted immediately.
d. The total time spent licking/biting the right hindpaw over the next 60 min is measured with a stopwatch and recorded to the nearest second in 5-min blocks. The early/acute phase is defined as 0-10 min post-injection, and the late/tonic phase as 10-60 min post-injection.IV- Hargreaves Test (HT, Plantar test)
a. Naïve mice are individually habituated for at least 2 hrs in a Plexiglas chambers (12.5 x 12.5 x 12.5 cm) atop a 3/16-inch glass floor (IITC Model 336).
b. Once motionless, a high-intensity projector lamp beam (setting = 3; approximately 45 W) is aimed at the plantar surface of the mid-hindpaw, 6 cm below the glass floor.
c. The latency to withdraw the hindpaw from the stimulus is measured automatically to the nearest 0.1 s.
d. Values represent the means of six such determinations on both paws over a 4-h testing period (no laterality effects are observed, nor any systematic effects of repeated testing).V- Hot Plate Test (HP)
a. Naïve mice are individually removed from their home cage and placed on a metal surface pre-heated and kept at 53.0 ± 0.2°C (IITC Model PE34M-HC) temperature.
b. The mice are enclosed within a transparent 20 cm high walls, 14 x 14 cm area Plexiglas chamber.
c. The latency to performing a hindpaw flutter/shake, lick, or jump (whichever occurred first) is measured to the nearest 0.1 s with a stopwatch.VI- Thermal Hypersensitivity Following Peripheral Nerve Injury (PNI_HT)
a. A set of naïve mice are subjected to a standardized peripheral nerve injury (Kim and Chung model) under gas anesthetic administration.
b. Soon after the mice reaches surgical anesthesia, the L4-L6 segmental spinal nerves are exposed distal to the dorsal root ganglion (DRG) and the L5 spinal nerve is tightly ligated with 6-0 silk thread about 5 mm distal to the DRG, taking special care to avoid possible damage to the L4 spinal nerve.
c. On the 7th day postoperatively, the group of mice with peripheral nerve injury are then tested on Hargreaves' test as described above.
d. Percent of total possible hyperalgesia is calculated by comparing hindpaw withdrawal latencies on the operated versus unoperated hindpaw.VII- Mechanical Hypersensitivity Following Peripheral Nerve Injury (PNI_VF)
a. Before a separate set of naïve mice are subjected to a standardized peripheral nerve injury (see above), they are initially tested for baseline mechanical sensitivity using calibrated von Frey monofilaments (as described below) for 7 consecutive days; wherein the preoperative threshold values are obtained by averaging the last 3 days of preoperative testing.
b. Following initial preoperative testing, as described above, the mice are given gas anesthesia before surgery is conducted.
c. Soon after the mice reaches surgical anesthesia, the L4-L6 segmental spinal nerves are exposed distal to the dorsal root ganglion (DRG) and the L5 spinal nerve is tightly ligated with 6-0 silk thread about 5 mm distal to the DRG, taking special care to avoid possible damage to the L4 spinal nerve.
d. On the 4th and 7th postoperatively days, the mice with peripheral nerve injury are again tested, this time for mechanical hyperalgesia using the same calibrated von Frey monofilaments (as described below).
e. Percent of mechanical hypersensitivity (allodynia) is calculated by comparing pre- versus post-operative hindpaw withdrawal thresholds.VIII- von Frey Threshold (VF)
a. Naïve mice are placed inside a transparent plastic box (4 x 4 x 7 cm) with a metal mesh floor.
b. Mechanical sensitivity is measured by determining the median 50% foot withdrawal threshold (measured in mN of discrete bending force), when a single prick to the hindfoot is applied steadily (>1 s duration) with a von Frey monofilament and via up-down method.
c. While mechanical sensitivity threshold of the stimulated hindpaw is measured for 7 consecutive days, only the average value of the last 3 days is reported.IX- Tail Withdrawal Test (TW49)
a. Naïve mice are individually removed from their home cage and gently restrained in a cloth/cardboard holder.
b. The distal half of the tail is immersed in heated water and maintained at 49.0 ± 0.2°C temperature, while the ambient temperature is kept at 22-23°C.
c. The latency to respond to heat stimulation vigorously and reflexively by withdrawing the tail is measured to the nearest 0.1 s with a stopwatch.
d. Three successive determinations,with interval of ≥20 s between testing, are averaged.Cold Ethanol Nociception and Tail Withdrawal Test (TW-15)
a. Thermostatically controlled immersion cooling device with ethanol is cooled and kept within 1°C of the target temperature (-15°C).
b. Another set of naïve mice are individually removed from their home cages and are lightly restrained and held in a cloth/ cardboard that they voluntarily entered.
c. Shortly after a mouse is restrained and totally immobilized (with the exception of its tail), the distal half of the tail is then immersed in cold ethanol.
d. Each mouse (n = 5-9 per strain) is tested only twice for cold sensitivity, at 5 min intervals and returned to its home cage in between latency determinations.
e. The time to vigorous withdrawal of the tail is measured to the nearest 0.1 sec with a stopwatch.
Hot Water Nociception and Tail Withdrawal Test (TW47.5)
a. A separate group of naïve mice from the same inbred strains are tested for hot water baseline latency to withdraw the the tail from circulating water held at 47.5 ± 0.5°C (TW47.5).
b. We chose to use a water temperature of 47.5°C,
c. 93-94 d old male breeders (n = 5-9 per strain), that have acclimated to the facility for at least 1 wk, are tested en masse within a 90 min period (2:15-3:45 P.M.).
d. Mice are tested exactly as in the TW-15 (see above), using the same test room and restrainer, except that their tails are now immersed in hot water maintained at 47.5 ± 0.5°C by a thermostatically controlled heater/ circulator pump.
e. Following each determination, the mice are likewise returned to their home cage.
f. Using a stopwatch, the latency to vigorously withdraw the tail from the hot waterbath is then measured to the nearest 0.1 s, and the mean of two determinations with an intertrial interval of at least 5 min is used in the analysis.
Submitting investigator's note: "Unlike in the CTW (TW-15) experiment, in which for reasons of practicality mice bred in our laboratory were tested in order of availability (but always in counterbalanced sets of three strains and always within 2 hr of midphotoperiod), the 47.5°C TW experiment was completely counterbalanced."
X- Thermal Hyperalgesia - Carrageenan (CAR_HT)
a. Carrageenan (2% 20 mg/mL) is freshly prepared, sonicated and suspended in normal saline.
b. Then 20 µL of the 2% carrageenan suspension is injected subcutaneously (s.q.) into the right plantar hindpaws of naïve mice using a 50 µL microsyringe with a 27-gauge needle.
c. After 4 ± .5 h post-injection, the mice are tested on the Hargreaves' test as described above.
d. Percent of thermal hypersensitivity is calculated by comparing withdrawal latencies on the injected versus uninjected hindpaw.XI- Bee venom test (BV, BVHT, BVCON)
a. Naïve mice are habituated to transparent Plexiglas cylinders (15 cm diameter x 46 cm high) on a glass floor for 30 min per day for 3 days.
b. Baseline thermal sensitivity measurements are conducted (see Hargreave's test above) the day before bee venom is injected.
c. Mice are fully restrained with cloth/cardboard and then injected with prepared bee venom solution in the left mid-plantar hindpaw.
d. Shortly after receiving injection, the mice are returned to the transparent Plexiglas cylinders for observation.
e. Total time spent within 60 min licking/biting the injected hindpaw is recorded (BV).
f. To measure thermal-induced hyperalgesia, mice previously tested for bee venom induced nociception (above) are also habituated for 2 h per day for 3 days in well-ventilated observation chambers (8.5 x 5 x 5.5 cm3) with transparent outer walls and ceiling, a 3/16th-inch-thick glass floor, and opaque inner walls that visually isolated them from each other.
g. After period of habituation, 2-4 h post-bee venom injection, a high intensity focused light beam is directed at the plantar surface of the mid-hindpaw of an inactive mouse.
h. Each mouse is tested 6 times on each hindpaw (ipsilateral and contralateral) with 5 min inter-trial interval; the mean of the 6 measurements is used for subsequent analysis.
i. Percent thermal hypersensitivity for each hindpaw (BVHT, BVCON) is calculated as: [(baseline latency - postinjection latency)/baseline latency] x 100.XII- Capsaicin test (CAP)
a. Naïve mice are habituated to transparent Plexiglas cylinders (30 cm diameter x 30 cm high) for 30 min.
b. After the mice are habituated to testing environment, they are restrained securely with a cloth/cardboard holder and injected in the right mid-plantar hind paw with freshly prepared solution of capsaicin.
c. Following capsaicin treatment, the mice are returned to the cylinders for subsequent observation.
d. The amount of time spent licking the injected paw (CAP) is measured with a stopwatch to the nearest 0.1 s for 15 min.Measurement of capsaicin-induced thermal hypersensitivity (CAPTW)
a. A separate group of naïve mice are taken from their home cages, placed securely in a cloth/cardboard holder for baseline measurement of tail-withdrawal test.
b. 0nce properly restrained, the distal half of their tail is immersed into a circulating water bath maintained at 47 ± 0.2°C, while the ambient temperature is kept at 22-23°C.
c. Using a stopwatch, the latency to vigorously withdraw the tail from the hot waterbath is measured to the nearest 0.1 s.
d. The mean of two determinations with an intertrial interval of at least 10 s is used in the analysis as the baseline value.
e. Shortly after baseline measurement of thermal-induced tail-withdrawal, the mice are cued for capsaicin-induced thermal hypersensitivity test.
f. Briefly as described above, the mice are securely restrained with a cloth/cardboard holder and injected with freshly prepared capsaicin subcutaneously (s.q.) into the dorsal surface and approximately midlevel of the tail.
g. Immediately after capsaicin treatment the mice are returned to their home cages.
h. The capsaicin-treated mice are removed from their home cages at 15, 30, 45, 60, 90, and 120 min postinjection, and securely restrained in a cloth/cardboard holder (see above).
i. 0nce properly restrained, the distal half of their tail is immersed into a circulating water bath maintained at 47 ± 0.2°C temperature.
j. Using a stopwatch, the latency to vigorously withdraw the tail from the hot waterbath is then measured to the nearest 0.1 s, and the mean of two determinations with an intertrial interval of at least 10 s is used in the analysis.
k. For each time point the percent hypersensitivity is calculated as: [(baseline latency + latency at time x)/baseline latency] x 100.
l. For the total percent hypersensitivity (CAPTW): the area under the percent hypersensitivity x time curve compared to the maximum possible hypersensitivity over the full time course is then calculated.
XIII- Intrathecal dynorphin-induced mechanical hypersensitivity (DYNVF)
Intrathecal (lumbo-sacral) injection of dynorphin is administered to evoke chronic mechanical hypersensitivity via a non-opods, N-methyl-d-aspartate (NMDA) receptor-mediated mechanism and allodynia as measured via von Frey test.
a. Before intrathecal administration of dynorphin, baseline mechanical threshold are measured using the von Frey test.
b. Naïve mice are placed inside a transparent plastic box (4 x 4 x 7 cm) with a metal mesh floor and are allowed to habituate for 1 h.
c. Mechanical sensitivity is measured by determining the median 50% foot withdrawal threshold (measured in mN of discrete bending force), when a single prick to the hindfoot is applied steadily (>1 s duration) with a von Frey monofilament and via up-down method.
d. While mechanical sensitivity threshold of the stimulated hindpaw is measured for 7 consecutive days, only the average value of the last 3 days is reported.
e. Subsequently awake, unanesthetized mice are securely and properly restrained for intrathecal injection.
f. Each mouse dorsal lumbo-sacral articulation is localized and marked.
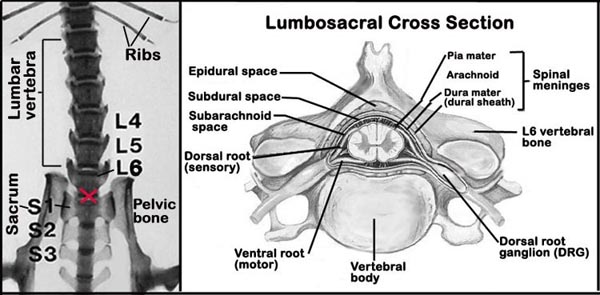
g. Because of the small size of the mouse, intrathecal space injection of freshly prepared dynorphin- using a small 30-gauge needle and microsyringe, require a great deal of expert precision (see Figure 1 below). This process, however, can be facilitated by the use of intrathecal guided apparatus.
h. Three days post-dynorphin injection, the von Frey test is re-administered.
i. Percent dynorphin-induced mechanical hypersensitivity is calculated as: [(baseline threshold - postinjection threshold)/baseline threshold] x 100.XIV- Tail-clip test (TC) - a modification of Haffner's tail-pinch test
a. Each naïve mouse is secured and properly restrained in a cloth/ cardboard holder so that a 1 cm diameter of the tail from the base is marked using a precision caliper.
b. Following marking of the tails, the mice are returned to their home cages and allowed to habituate for 30 min.
c. Shortly after habituation, the mice are again secured and properly restrained in a cloth/ cardboard holder for the application of an alligator clip to the1 cm-marking from the base of the tail .
d. In order to exert 500 x g of mechanical force, rubber cuffs are wrapped around the teeth of each alligator jaw.
e. After the application of the tail-clip, each mouse is immediately removed from the holder and placed in an observation chamber.
f. The latency to lick, bite, grab, or bring the nose to within 1 cm of the alligator clip is measured with a stopwatch to the nearest 0.1 s, after which the clip is immediately removed.
g. To prevent the possibility of tail injury, the alligator clip is removed after 60 s.All strains except CBA and DBA/2 are tested in this assay, a slight modification of the classic tail-flick test of D'Amour and Smith (1941). These data are collected as baseline values in a published inbred mouse strain survey of electro-acupuncture analgesia (Wan et al., 2001).
a. Briefly, mice are restrained in well-ventilated, cylindrical Plexiglas restrainers, with the tail extended from one end.
b. After insertion of acupuncture needles in the hind legs, the mice are allowed to habituate for 30 min (with no current applied),
c. After habituation, a focused light from a 12.5-W projection bulb is applied directly to the middle of the tail.
d. Soon after the mouse flicked its tail a digital timer connected in series measured the tail-flick latency to the nearest 0.1 s.
e. If the mouse does not respond within 10 s, the bulb is turned off to avoid the possibility of tissue injury.
f. Three baseline threshold measurements are taken at 5-min intervals and the mean value calculated (TF).Preparation for acupuncture needle insertion
As much as practically possible based on mouse availability, multiple strains and both sexes are represented in each testing session.
a. Naïve mice are restrained in 8-cm long, cylindrical Plexiglas restrainers, and partially immobilized within by properly placed cotton balls over the sacral region.
b. Small holes are placed in the rostral end of the restrainer to provide ventilation.
c. The hind legs of the mouse are extended outward distally through the two rear openings.
d. After the mouse entered the restrainer, a round metal piece is used to cover the rear entrance, except for a hole out through which the mouse tail is extended.
e. With the mouse securely fixed, the acupuncture needles are inserted into the hind legs and the freely accessible tail is prepared for nociceptive testing.
f. In order to test eight mice simultaneous per session, properly restrained mice are positioned to the top of a rack suspended 25 cm above a table top.Insertion of acupuncture needles
a. Each stainless steel acupuncture needle (0.15 mm diameter) is bent into an `L' shape.
b. The proximal end is left unsoldered and not connected to an electric stimulator for baseline tail flick determination.
c. The bent distal ends of two needles per mouse are inserted into accupoints on the hind leg:
__(1) Zusanli (ST 36), near the knee joint, 2 mm lateral to the anterior tubercle of the tibia (see Figure 2A);
__(2) Sanyinjiao (SP 6), near the ankle joint, at the level of the superior border of the medial malleolus between the posterior border of the tibia and the anterior border of the Achilles tendon (see Figure 2B).
d. Needles are inserted 3 mm in depth in ST 36 and just penetrable in SP 6.
e. After insertion, the needles are fixed in situ with adhesive tape.Thermal nociception and tail-flick test
a. Shortly after the mice are habituated to the placement of the acupuncture needles (see above) and the restrainer for at least 30 min, a focused light from 12.5 W projection bulb is applied directly to the middle of the tail (3 mm diameter).
b. The projection bulb is turned off as soon as the mouse flicked its tail, and a digital timer connected in series measured the tail-flick latency (TFL) to an accuracy of 0.1 s.
c. During the experiment, room temperature is carefully monitored and maintained at 22-1°C, to minimize the possible influence of ambient temperature.
d. A cut-off latency of 10 s is used in order to avoid the possibility of tissue damage to the superficial tail.
e. Then, tail flick latency is assessed three times at 5-min intervals and the mean value of the three assessments is taken as the basal nociceptive threshold.Figure 1. Schematic diagram of the spinal column (L = lumbar, S = sacral) showing the lumbo-sacral region (red X) and the intrathecal region within the spinal meninges.
Data collected by investigator
Chemically (formalin)-induced pain response durations in the early and late phase of the formalin test. Formalin-induced hypersensitivity to capsaicin, bee-venom, acetic acid, and magnesium sulfate stimulation.
Thermally-induced pain responses to hot plate test, Hargreaves test, and tail flick test. Thermal-induced hypersensitivity to capsaicin, bee venom (both ipsilateral and contralateral), carrageenan. Peripheral nerve injury-induced sensitivity to hot stimulation. Tail withdrawals at 49°C and 47.5°C heat and -15°C cold temperatures.
Mechanical nociception to tail clip, and von Frey test. Mechanical hypersensitivity to von Frey test following nerve injury and dynorphin treatment.
autotomy: the transection or ligation of a nerve (i.e. sciatic nerve) preventing regeneration.
allodynia: evoked pain from an innocuous stimulus.
capsaicin: the alkaloid ingredient in hot peppers that is pungent ("hot" to taste) and irritant.
carrageenan (lambda): is a mucopolysaccharide that is obtained from Iris sea moss (Chondrus); when injected subcutaneously produces acute hypersensitivity and inflammation that is effectively maxed 3-5 h post-injection. This nociceptive effect is readily reversible with the administration of steroidal (i.e. corticosteroids) and non-steroidal anti-inflammatory analgesic drugs.
intrathecal: within the spinal cord meninges (pia mater, arachnoid, dura mater).
Hargreaves test: also known as plantar test.
nociception: pain sensation.
References
Callahan BL, Gil AS, Levesque A, Mogil JS. Modulation of mechanical and thermal nociceptive sensitivity in the laboratory mouse by behavioral state. J Pain. 2008 Feb;9(2):174-84. Epub 2007 Dec 21.
PubMed 18088557 MartÃnez V, Thakur S, Mogil JS, Taché Y, Mayer EA. Differential effects of chemical and mechanical colonic irritation on behavioral pain response to intraperitoneal acetic acid in mice. Pain. 1999 May;81(1-2):179-86.
PubMed 10353506 Mogil JS, Adhikari SM. Hot and cold nociception are genetically correlated. J Neurosci. 1999 Sep 15;19(18):RC25.
PubMed 10479718 Wall PD, Devor M, Inbal R, Scadding JW, Schonfeld D, Seltzer Z, Tomkiewicz MM. Autotomy following peripheral nerve lesions: experimental anaesthesia dolorosa. Pain. 1979 Oct;7(2):103-11.
PubMed 574931 Wan Y, Wilson SG, Han J, Mogil JS. The effect of genotype on sensitivity to electroacupuncture analgesia. Pain. 2001 Mar;91(1-2):5-13.
PubMed 11240073