ITP1 project protocol
Interventions Testing Program: Effects of various treatments on lifespan and related phenotypes in genetically heterogeneous mice (UM-HET3) (2004-2025)
Miller RA, Strong R, Korstanje R, Harrison DE, Rosenthal NAProject protocol - general information
See publications for specific information about individual studies.Introduction
The ITP is a multi-site collaborative effort with the goal of identifying compounds that promote healthy aging in a genetically heterogeneous mouse model.
The main goals are to:
- Provide preliminary evidence on compounds that have translational potential for human use to improve human healthspan with age.
- Provide evidence for pathways that influence aging and are therefore targets for interventions.
The use of triplicate testing sites is a key aspect of the ITP testing paradigm:
- University of Michigan (UM), Ann Arbor Michigan USA, PI Richard Miller
- The Jackson Laboratory (TJL), Bar Harbor Maine USA, PI David Harrison
- University of Texas Health Science Center at San Antonio (UT), San Antonio Texas USA, PI Randy Strong
Test compounds
MPD houses data for a subset of ITP studies (more data will be added over time). The compounds and their rationale for testing are listed here.
The ITP program engages the broader research community in the search for agents that slow aging and extend mouse lifespan. An annual call for proposals is made to the research community. For more information, see the NIA-ITP website.
Experimental design
Two-stage program:
- Stage 1: Focuses on lifespan as the primary endpoint.
- Stage 2: Studies follow-up on positive findings from Stage 1 with additional lifespan studies, dose-response studies, a wide array of healthspan measurements and cross-sectional pathology analysis.
The mouse model for all ITP studies is the UM-HET3 four-way cross as illustrated below.
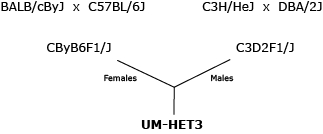
While no two four-way cross mice are identical, each mouse shares half of its genome with every other mouse in the population, and the population-level diversity can be replicated at any time by starting with the same parental strains. In terms of nuclear genome, all UM-HET3 mice are full sibs.
Basic husbandry SOPs were developed to ensure consistency at the three test sites with minimal variation. See Animal documentation.
Breeding is done at each of the three test sites to produce UM-HET3 mice. The mice for each year's testing are bred over 6-8 months to minimize cohort effects.
For Stage 1 longevity protocols, each test site has 44 male and 36 female offspring in each treatment group (males are oversampled because of the losses expected due to fighting). The control group at each site has twice the number of mice (88 males and 72 females), to provide superior statistical power at a cost lower than would be required for a balanced design.
Each cage is assigned to treatment or control groups using a random number table to prevent bias from influencing assignment.
In the absence of scientific or logistical factors, the default protocol is to begin treatment when the mice turn 4 months old. There are exceptions (see age at initiation)
Test compounds are administered in the diet using an NIH31-based formulation. Batches of food are prepared by TestDiet, Inc. (Richmond, Indiana USA) to meet the needs of all three test sites for 4-month intervals so that all sites use the same batches of food. Control and treatment diets are based on Purina 5LG6. Diets are routinely tested by UT to ensure that the food contains the expected amount of the test compound.
All three test sites start the experimental diets at the same age (+/- 3 days), which is determined per diet and has varied from 4 to 16 months of age.
Body weight is measured at 6, 12, 18, 24 months of age. Mice may be permanently removed from the lifespan study for physiological or behavioral testing at particular ages (see below).
Cages are checked daily for health, and mice are euthanized when an animal appears moribund (unlikely to survive another 2 days). Classification of moribund status is based on the appearance of at least two of the following clinical signs:
- Severe lethargy, as indicated by reluctance to move when gently prodded with forceps.
- Inability to eat or drink.
- Severe balance or gait disturbance.
- Rapid weight loss over a period of 1 week or more.
- An ulcerated or bleeding tumor.
Entire cages of mice are culled if there is excessive fighting leading to open wounds covering 20% or more of an animal's skin.
The endpoint is natural death, except in the case of those animals that are:
- Removed for fighting.
- Removed for humane reasons.
- Removed for physiological or behavioral testing.
- Lost because of a technical mistake or accident.
Removed animals are euthanized.
Longevity mice that die at ages older than 600 days (~19 mo) are routinely preserved in fixative for possible future necropsy study, using a standardized protocol across test sites.
Health monitoring: Each colony is evaluated four times per year at each test site for infectious agents by serological assessment of sentinel animals.
Care and use: All procedures are approved by each test site's Institutional Animal Care and Use Committee and follow the guidelines set forth by the NIH (Guide for the Care and Use of Laboratory Animals).
See publications for specific information about individual studies.