Harrill1 project protocol
Drug study: Clinico-pathological survey of the liver and adverse reactions in response to isoniazid treatment in females of 34 inbred strains of mice (2011)
Harrill A
Project protocol — Contents
Workflow and sampling
Equipment, supplies, softwares
Reagents, solutions
Procedure: Study design treatment essentials
Procedure: Necropsy and blood collection via vena cava
Procedure: Preparation for serum ALT measurements
Procedure: Measurement of hepatic triglyceride and cholesterol
Procedure: Liver histopathology
Definitions
Data
ReferencesWorkflow
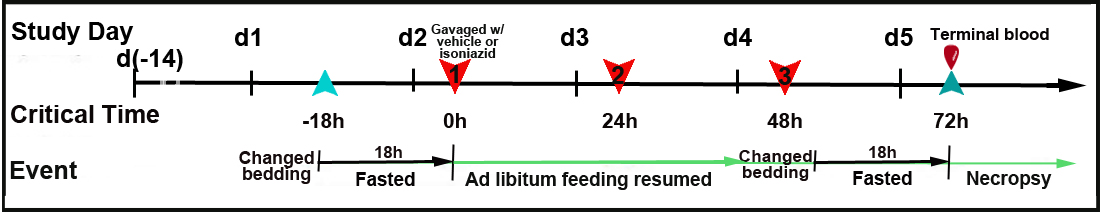
Mice fasted for 18 h refer to Figure 1 Mice weighed approximately 4 days before treatment and subsequently after each treatment Mice gavaged with isoniazid (100 mg/kg) or vehicle for 3 consecutive days Ad libitum feeding resumed following treatment Mice fasted for 18 h before necropsy Mice euthanized, weighed, and terminal blood collected for ALT Necropsy conducted, liver and spleen harvested and weighed; liver samples collected for histology and liver lipid assay Liver samples processed for histopathology and liver lipids Histopathology samples scored for isoniazid toxic effects See Abbreviations
Equipment, supplies, softwares
- ProvantisTM 8 (Instem ProvantisTM; Conshohoken, PA) data collection system
- Balance scale
- Gavaging needles for mice
- Dissecting tools
- -80 °C Freezer
- Blood collection serum separation tubes
- Safe-lock tubes
- 96-well microtiter plates
- Microtiter plate (spectrophotometer) reader
- Tissue homogenizer
- 80-100°C water bath
- Centrifuge
- Microtome for histology
- Microscope with 100x objectives and image capture capability
- Microscrope slides
- CO2 gas euthania setup
- Isoniazid (Fisher Scientific, Pittsburgh, PA) dissolved in sterile, distilled water and prepared fresh before use
- Vehicle: sterile distiled water
- Anesthesia: pentobarbital sodium (Nembutal, Abott Laboratories)
- Triglyceride Quantification Kit (Abcam)
- 5% Triton-X100
- Tissue fixative: 10% neutral phosphate buffered formalin
- Tissue-staining: Hematoxylin and Eosin stain (H & E)
- Antech Diagnostics GLP (Morrisville, NC) for serum enzyme analysis of alanine aminotransferase (ALT)
Acclimation to test conditions
Mice are acclimated to housing conditions for at least a week.
Procedure: Study design and isoniazid treatment essentials
a. Mice receiving vehicle or isoniazid are randomized using ProvantisTM 8 to ensure equal mean body weights in each treatment group.
b. Immediately prior to fasting, cage bedding is changed to eliminate ingestion of feed crumbs or coprophagy.
c. To deplete glutathione reserves within the liver (Harrill et al., 2009), mice are fasted for 18h on Day 1.
d. Baseline body weight is recorded before the first isoniazid or vehicle (sterile, distilled water) treatment is administered, and ad libitum feeding is resumed.
e. The same time in the mornings (0900 ± 1.5h) of Days 2, 3, and 4 (t=0h, t=24h, t=48h) mice are treated with either isoniazid or vehicle to avoid any diurnal effects (Fig. 1).
f. On Day 2 (t=0h) mice are gavaged with isoniazid at a dose of 100 mg/kg body weight or with vehicle at a volume of 10 mL/kg (Fig. 1). Mice are dosed on Days 3 and 4 under similar conditions.
g. Following a change in cage bedding, mice are fasted again for 18h before necropsy to reduce hepatic glycogen, thereby facilitating histopathological assessment of liver injury. Mice are sacrificed 24h after the final dose on Day 5 (t=72h following initial dosing) of the study.Oral gavaging technique
Gavaging is used to dose mice with a specified volume (10 mL/kg) of isoniazid or vehicle directly into the stomach. Only specialized, commercially available gavage needles for mice are used. The syringe is first filled with the appropriate volume and material before the needle is attached.
a. A mouse is gently restrained by the scruff.
b. The tip or ball of the gavaging needle is placed into the mouse's mouth and then gently slid past the back of the tongue. Note: The tip of the needle should slide easily down the esophagus, otherwise, the needle is improperly placed and should never be forced. Any resistance encountered requires the removal of the needle and followed by reinsertion.
c. Further injury to surrounding tissues is carefully avoided by holding the syringe and preventing it from aspirating.
d. Only when the needle is properly placed, a given dose of vehicle or isoniazid is administered via the attachment of the syringe.Procedure: Necropsy and blood collection via vena cava
a. On Day 5, fasted mice are weighed to determine anesthetic dose (pentobarbital sodium 150 mg/kg body weight) prior to necropsy.
b. Terminal blood is collected via the vena cava while under general anesthesia.
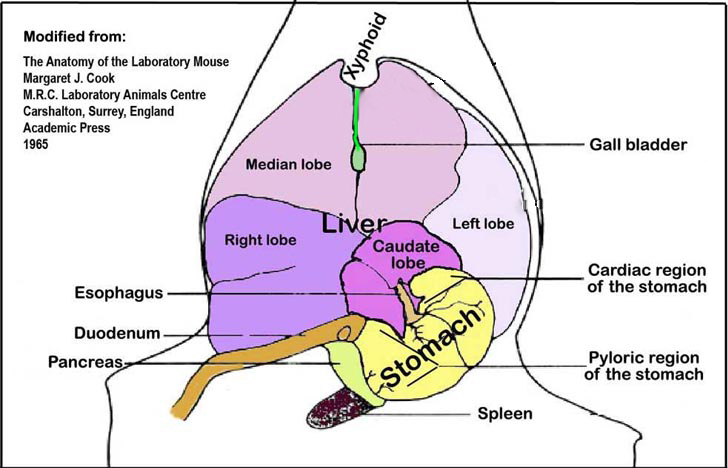
c. Following exanguination, spleen and liver are harvested and weighed.
d. Sample sections of the median and left lobes are fixed in 10% buffered formalin for histology.
e. Sample sections of the left liver lobes (Fig. 2) are flash frozen in liquid nitrogen, stored in safe-locked tubes, and then sored at -80°C freezer until anaylsis.Procedure: Preparation for serum ALT measurement
a. Blood samples are allowed to clot at room temperature for at least 1h.
b. Serum is separated from blood cells using serum separation tubes and a table top centrifuge at a speed of 13,000 x g for 20 min.
c. The serum layer is pipetted carefully without disturbing the packed blood cell layer below and then transferred into a new pre-labeled tube and stored at -80°C until ready to be assayed.
d. The frozen serum is sealed in plastic bags and packaged in wet ice for transport to Antech Diagnostics GLP (Morrisville, NC), which performed the clinical chemistry analyses.
e. Serum is assessed for ALT enzyme level.Procedure: Measurement of hepatic triglyceride and cholesterol
a. Hepatic triglycerides are quantified using the Triglyceride Quantification Kit according to the manufacturerâs recommendations.
b. Briefly, 100 mg liver tissue is homogenized with 1 mL 5% Triton-X100 in water, and then slowly heated to 80-100°C in a water bath for 2-5 min, or until the Triton X-100 becomes cloudy, at which time it is slowly cooled down to room temperature.
c. The heating process is repeated one more time to solublize all triglyceride into solution.
d. The liver homogenate samples are centrifuged for 5 min to remove any insoluble materials.
e. The pelleted precipitates are discarded and the supernatant is diluted 10 fold with dH2O.
f. Enough Triglyceride Reaction Mixture, consisting of 46 µL Triglyceride Assay Buffer, 2 µL Triglyceride Probe, and 2 µL of Triglyceride Enzyme Mix, or a total of 50 µL reagent mix for each well in a 96-well plate is prepared and applied.
g. 50 µL of diluted sample (or diluted standard) is added to each well for a final volume of 100 µL and mixed well.
h. Light-protected 96-well microplates are incubated at room temperature for 1h.
i. Colorimetric reading for O.D. is done at 570 nm using a microtiter plate reader. Reaction mixture is stable for at least 2h.
j. Hepatic triglyceride concentration is plotted using a standard curve method.
k. Liver is frozen in liquid nitrogen until processed for hepatic determination of cholesterol. Tissue is homogeneized, lipids extracted through a Folch procedure and hepatic cholesterol is determined using cholesterol oxidase (Sigma) while free cholesterol is determined by high pressure liquid chromatography (HPLC) as previously described (Lammert et al. 1999; Wang et al. 1999). Cholesterol esters are calculated by substraction of free cholesterol from total cholesterol.Procedure: Liver histopathology
a. After 24-48h of 10% neutral buffered formalin (NBF)-fixation, the median lobe is further trimmed and placed in tissue cassettes and returned to 10% NBF.
b. After an additional 24-48h of fixation, formalin-fixed tissue cassettes are transferred into 70% EtOH solution.
c. For each mouse a paraffin-embedded block is prepared from the tissue cassette.
d. Embedded liver tissues are cut to a thickness of 5 µm sections and applied to a glass slide in duplicate.
e. The sections are then stained with H&E.
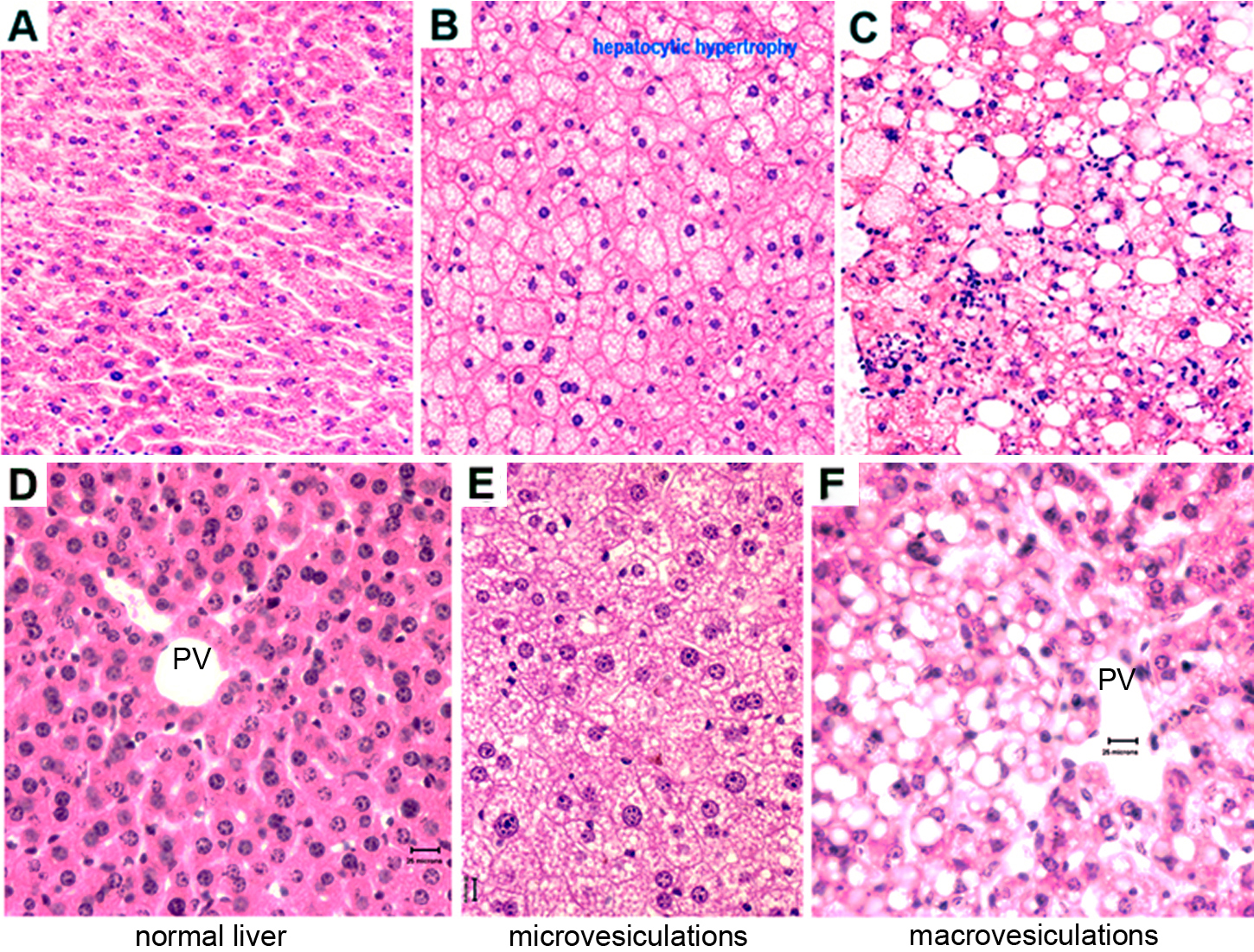
f. Each slide or histopathology sample is scored blindly for liver injury (Fig. 3).
g. A veterinary pathologist independently verified the liver histopathology scores.16 morphological diagnoses: Scored markers of liver injury
Severity scores: 1 = minimal; 2 = slight-mild; 3 = moderate; 4 = moderately severe; 5 = severe-high
1. Multifocal, portal, lymphohistiocytic infiltrate
2. Multifocal lymphohistiocytic infiltrate
3. Focal lymphohistiocytic infiltrate
4. Multifocal hepato-cytoplasmic microvesiculation
5. Diffuse hepato-cytoplasmic microvesiculation*
6. Multifocal hepato-cytoplasmic macrovesiculation*
7. Diffuse centrilobular hepatic hypertrophy
8. Diffuse panlobular hepatic hypertrophy
9. Focal mixed cell infiltrate
10. Multifocal mixed cell infiltrate*
11. Multifocal hepatic apoptosis
12. Focal hepatic necrosis
13. Multifocal hepatic necrosis
14. Focal hepatic thrombosis
15. Chronic, multifocal phlebitis
16. Multifocal extramedullary hematopoiesis
* See Figure 3 for photomicrograph examples
Figure 2. An illustration of the liver lobes and the spleen and their orientation within the abdominal cavity viewed ventro-dorsally.
Figure 3. A photomicrograph example of liver histopathology and morphological evaluation. Panels A and D: normal liver histology. Panels B and E: diffuse hepato-cytoplasmic microvesiculations (steatosis). Panels C and F: multifocal hepato-cytoplasmic macrovesiculations (fatty change). Note that in Panel C there are visible mixed cell infiltates. Though Panels A-C and Panels D-F were processed at different times, both were fixed in Bouin's fixative. PV=potal vein
alanine aminotransferase hematoxylin and eosin anti-tuberculosis drug, known idiosyncratic drug-induced liver injury high performance liquid chromatography drug-induced liver injury optical density in a colorimetric assay triglyceride ethanol cholesterol neutral-buffered formalin Correct background by subtracting the value derived from the 0 triglyceride standard from all sample readings. Plot the standard curve. Apply sample readings to the standard curve. Triglyceride concentration can then be calculated:
C = Ts / Sv nmol/µL or µmol/mL or mM
Where:
C = triglyceride concentration
Ts is triglyceride amount from standard curve (nmol)
Sv is the sample volume (before dilution) added in sample wells (µL)Converting raw data in 0.3 nmol/µg ====> triglyceride mg/g liver tissue:
Given: molar mass of triglyceride standard = 885.43 g/mol
Calculation: (0.3 nmol/µg) X (885.43 X µg/nmol) = 265.6 X mg/g = 265.6 mg/g liver sample
Converting raw data in nmol/µg ====> triglyceride mg/g liver tissue with a liver sample of 0.1g:
Tissue preparation: determine sample mass/volume by homogenizing 100 mg liver sample in 1 mL of 5% Triton-X100 in water and diluting the homogenate 10-fold with distiled water. The resulting sample concentration would then be 0.01 g/mL (mass of tissue/sample volume).
Calculation: raw triglyceride data of (5 µmol/mL) ÷ (0.01 g/mL) = 500 µmol/g tissue
Data collected by investigator
- body weight at days 1, 2, 3, and 4 in control and isoniazid treated mice
- blood enzyme serum ALT in control and isoniazid treated mice
- liver weight and liver weight as percent of body weight in control and isoniazid treated mice
- liver pathology scores in control and isoniazid treated mice
- hepatic CHOL and hepatic TG in control and isoniazid treated mice
- spleen weight and spleen weight as percent of body weight in control and isoniazid treated mice