Gould1 project protocol
Drug study: Antidepressant-like responses to lithium in males of 8 inbred mouse strains and 3 outbred stocks (2011)
Can A, Gould TDWith: Blackwell RA, Piantadosi SC, Dao DT, O'Donnell KC
Project protocol — Contents
Workflow and sampling
Equipment
Reagents, supplies, and solutions
Procedures
Data
References
Workflow
(mg LiCl/kg BW)
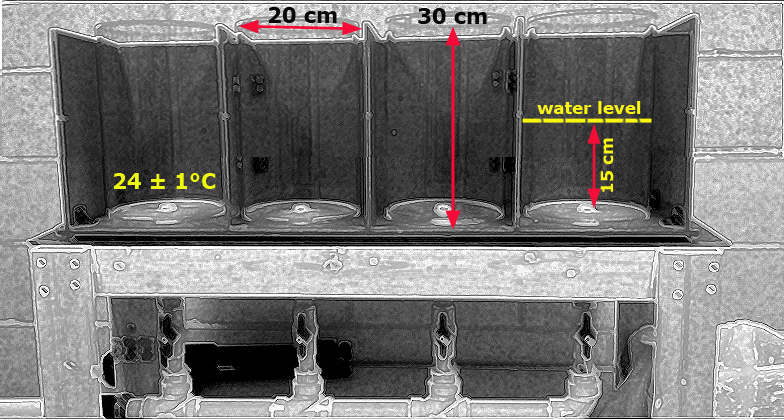
- Plexiglas cylinders (30 cm height à 20 cm diameter) filled with 15 cm of tap water at a temperature of 24 ± 1°C equipped with and videotape monitoring capability
- Balance scale
- Small rodent dissecting kit
- Freezer -80°C
- Centrifuge
- Polytron homogenizer (Kinematica AG Model PCU 11; Littau, Switzerland)
- Digital flame photometer (Cole-Palmer Model 2655-00; Chicago, IL)
Reagents, supplies, solutions Â
- paper towels
- isoflurane gas anesthesia setup
- 0.9% saline
- lithium chloride (LiCl) (Sigma, Saint Louis, MO, USA)
- 1 and 5 mL syringes
- 16 and 19 gauge needles
- centrifuge tubes
- 0.5N trichloroacetic acid
- freezer-safe (-80°C) storage containers for serum and brain samples
I. Lithium administration
a. Lithium chloride (LiCl) solutions are freshly made by dissolving LiCl in 0.9% saline.
b. 5 h before behavioral tests begin, mice are weighed and then given i.p. injections of either saline, 200, 300, or 400 mg/kg LiCl in an injection volume of 4 mL/kg body weight.
c. Mice are also given, in addition to their existing water bottle, a 0.9% saline bottle to reduce possible ion imbalances caused by lithium.
II. Behavioral forced swim test (FST)
Lithium tends to have a mood stabilizing, antidepressant effect in certain individuals with depression and/or bipolar disorder. To test this phenomena in mice and explore possible genetic basis of differential sensitivity, FST is used in 11 diverse strains in combination with different doses of lithium. Water exposure is one of the most anxiety provoking stimuli for mice. When treated, lithium-sensitive and anxious mice will likely continue to struggle and swim to escape, consequently showing less "behavioral despair" or immobility.
a. The FST is conducted using Plexiglas cylinders filled with 15 cm of tap water maintained between 23 - 25°C temperature.
b. After 5 h of lithium administration, mice are tested and videotaped during a 6-min FST session. All mice within each strain are represented in each group tested and their order are counterbalanced and/or randomized.
c. Video recordings are subsequently analyzed for activity during FST.
d. The last 4 min of each session are scored for mobility/immobility by a trained observer who is blinded to the group assignments. Mobility is defined as any movement beyond what is necessary to maintain the head above water.
e. At the end of the trial session, mice are taken out of the water, dried with a paper towel and placed back in their home cages.
III. Lithium concentration measurement
a. Following behavioral tests, mice are anesthetized using isoflurane.
b. Once mice are fully anesthetized, intra-cardiac blood is collected using 16G needle and 5 mL syringe.
c. Following cardiac exanguination, brains are dissected, weighed, and frozen at -80°C for later analysis.
d. Fully thawed brains are homogenized using a polytron in three volumes of 0.5 N trichloroacetic acid for denaturation.
e. Brain samples are then centrifuged and the supernatant is evaluated for lithium levels (mmol/kg, wet weight) using a digital flame photometer.
f. Blood samples are allowed to clot at room temperature for at least 30 min, and then centrifuged to collect serum samples.
g. Serum lithium levels (mmol/L) are determined using the same digital flame photometer above.
Figure 1. Schematic illustration of an FST setup.
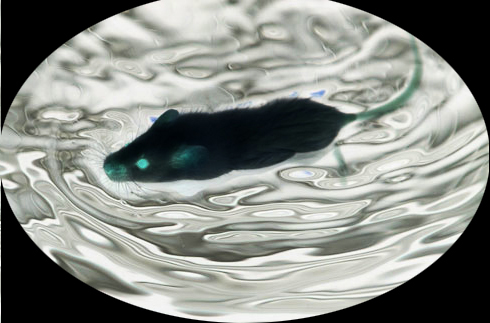
Figure 2. Videotaped image of a mouse during FST.Data collected by investigator
• duration of immobility following forced swim test with controls
• serum lithium concentration
• brain lithium concentration
References
Gould TD, Dao DT, Kovacsics CE. The open field test. In: Mood and Anxiety Related Phenotypes in Mice: characterization using behavioral tests (book), Gould TD editor, Humana Press, pp 1-20. (2009).
Shim SS, Gould TD. Neuroprotective and neurotrophic actions of lithium: implications for the treatment of bipolar disorder, Alzheimer's disease and neurodegenerative disorders. (in press). In: Metals and Neurodegeneration (book), Huang S editor, Research Signpost, Kerala, India.