DiCurzio1 project protocol
Adrenal gland weight and morphology in 64 BXD recombinant inbred strains of mice (2010)
Di Curzio DL, Goldowitz DProject protocol — Contents
Workflow and sampling
Equipment
Reagents, supplies, and solutions
Procedures
Data
References
Workflow
• Fume hood
• Zeiss dissecting microscope
• Rodent dissecting kit
• Mettler Toledo balance scale
• Leitz rotating microtome (Leitz 1512; Leitz, Wetzlar, Germany).
• Zeiss light microscope and Zeiss Axiovert 200 inverted light microscope with a Zeiss AxiocamHR color camera attached to a computerized Zeiss Axiovision 4.6 imaging system (Zeiss, Jena, Germany)
• Microscope objective lens: 5x and 10x
• Leitz rotating microtome (Leitz 1512; Leitz, Wetzlar, Germany)Reagents, supplies, solutions Â
• Glass storage vials (5-10 mL)
• Bouinâs fixative (75 mL picric acid, 25 mL 37% formalin, 5 mL glacial acetic acid)
• Cleaning and disinfectant supplies
• paraffin
• Hematoxylin and eosin (H&E) stain
• Superfrost Plus slides (Fisher Scientific, Hampton, NH, USA)
I. Dissection of the adrenal glands
a. Mice are housed temporarily in a fume hood up to 24 h before dissection.
b. On the day of dissection, cages are removed from the hood one at a time.
c. The individual cages are placed on a cart on the far end of the room, separated from the dissection area.
d. Mice are removed from each cage, while their body weight, coat color, sex, birth, and histology dates are noted, and then placed in a separate new cage. This is performed calmly so as to minimize the possibility of sympathetic nervous system activation, which might ultimately affect adrenal weights.
e. After 10 min, each mouse is subjected to cervical dislocation and the abdominal cavity opened to provide access to the whole kidneys, which are removed one at a time with the adrenal glands attached.
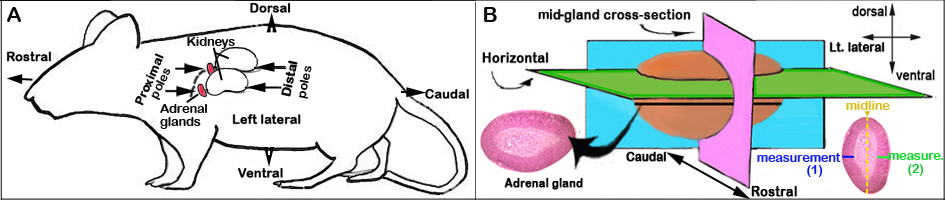
f. The adrenal glands are visually identified with their lighter flesh-color compared with the surrounding relatively darker kidney tissue. The adrenal glands are situated on the proximal pole of the kidneys (see Figure 1 below).
g. A Zeiss dissecting microscope is used to facilitate dissection of the adrenal gland from the surrounding kidneys and connective tissue.
h. Dissected adrenal glands are then weighed on a Mettler Toledo balance scale to the 0.1 mg accuracy, placed in vials with Bouinâs fixative and stored overnight for subsequent histology (see details below).II. Adrenal glands histology preparation
a. After overnight fixation, the adrenals are embedded in paraffin blocks and positioned in a horizontal/longitudinal plane for sectioning (see Figure 1B below). Two to five adrenals are embedded in each block and placed together according to histological date and by sex.
b. The embedded adrenal glands are sectioned at 8 µm using a Leitz rotating microtome.
c. Two consecutive sections out of every 20 are mounted on Superfrost Plus slides and stained with H&E for histological observation.
d. After identifying the section that containes the most extensive region of each adrenal, additional sections are mounted ±10 µm from this section and stained with H&E.
e. These latter sections are examined to select the section with the largest adrenal area from which structural and morphometric measurements are later performed (see details below).III. Morphometric examination of adrenal histology
a. All adrenal gland sections are analyzed using bright-field light microscopy.
b. The adrenal width measurements are taken at the approximate "midline" where the width of the adrenals is the greatest across the horizontal sections analyzed.
c. Morphometric examination is conducted using an inverted light microscope with a color camera attached to a computerized imaging system. The examination includes quantitative measurements of the adrenal cortical zones and medulla width.
d. For the total width and medulla measurements, images of the adrenal sections are taken using a 5x objective.
e. To obtain more precise measurements of the cortical regions, the tissue is viewed with a 10x objective and optovar setting at 1.60.
f. The width of each cortical region is measured along the midline, with the cortical zone thickness being averaged for the measurements taken on either side of the medulla (n = 2). Under optimal conditions, H&E staining provides visible morphological borders between each of the cortical zones and the medulla (see DiCurzio et al., 2011).
NOTES:
Figure 1. Schematic orientation of the adrenal glands. (A) in situ and (B) ex-vivo (not drawn to size).
- In the age range tested, all females and some males (strain-specific) had visible X-zones (see ‘Xzone_presence’).
- Some females but not males exhibited lipoid structures (lipid-filled cells undergoing fatty degeneration and vacuolation) in the cortex (see ‘lipoid_presence’).
- Lipoid structures have been suggested to be part of the degenerating X-zone. However X-zone boundaries could not always be identified encapsulating the lipoid structures.
- If lipoid structures were clearly found within the X-zone boundaries, then lipoid structures along the midline were included in the X-zone measurements.
- If lipoid structures were not clearly flanked by X-zone on both sides, they were not included in the X-zone measurement (or any other cortical measurement).
- These data show that there is a statistically significant difference in X-zone widths between females that contain versus do not contain lipoid structures.
- These data show that for females containing lipoid structures, there is no statistically significant difference between X-zone widths that include versus exclude lipoid structures in the measurement.
- Comments regarding the lipoid zone were provided by the investigator. (lipoid structures within the X-zone were described in terms meaning “dispersed” and/or “scant”; otherwise, lipoid structures were described as “surrounding the X-zone”.
- MPD standardized terms describing lipoid structures are available in the Supplementary data file.
Data collected by investigator
• Body weight at testing and left and right adrenal weights
• Whole adrenal and medullary widths
• Thickness of each adrenal cortical zone: zona glomerulosa, zona intermedia, zona fasciculata, zona reticularis, X-zone
References
Deschepper CF, Olson JL, Otis M, Gallo-Payet N. Characterization of blood pressure and morphological traits in cardiovascular-related organs in 13 different inbred mouse strains. J Appl Physiol. 2004 Jul;97(1):369-76. Epub 2004 Mar 26.
PubMed 15047670