Bopp1 project protocol
Susceptibility to infection with Plasmodium berghei (malaria) in 31 inbred mouse strains (2010)
Bopp SE, Ramachandran V, Wiltshire T, Winzeler EAWith: Henson K, Luzader A, Lindstrom M, Spooner M, Steffy BM, Suzuki O, Janse C, Waters AP, Zhou Y
Project protocol — Contents
Workflow and sampling
Equipment
Reagents, supplies, and solutions
Procedures for measuring susceptibility to Plasmodium berghei malaria infection
Data
References
Workflow
Tail blood is collected from moribund mice and fixed Blood is analyzed for schizont containing erythrocytes Each mouse is anesthetized and given D-luciferin i.p. Individual organs are dissected and imaged for bioluminescence • Bio Medic Data Systems, Inc. (BMDS) implantable microchip system, specifically the DAS-5002 Notebook System and the IPTT-300 Implantable Programmable Temperature Transponders. The DAS-5002 Notebook System is a portable hand-held reader-programmer used to program and scan BMDS temperature microchips.
• FACStar plus cytofluorometer (Becton Dickinson) equipped with a Coherent Innova 90 laser tuned to UV excitation (351 nm, 200 mW)
• Intensified-chargecoupled device (I-CCD) photon counting video camera of the in vivo Imaging system (IVIS 100, Xenogen)
• Visualization programs LIVING IMAGE (Xenogen) and IGOR-PRO (Wavemetrics)
Reagents, supplies, solutions Â
• The Plasmodium berghei ANKA strain PbGFPâLUCSCH expresses a GFP-luciferase fusion protein under the schizont specific promoter of the P. berghei ama1 gene. The fusion protein is expressed in the schizont stage but also in the very early ring stage. Parasites from frozen stocks of this strain are propagated and maintained in BALB/cByJ mice. The parasites are preserved in Alseverâs solution containing 10% glycerol and stored in liquid nitrogen.
• isoflurane gas anesthesia setup
• 16-gauge needle microchip placement device
• Bio Medic Data Systems (BMDS) implantable microchips
• razor blades
• 0.25% glutaraldehyde in PBS (pH 7.4)
• 4°C refrigerator or ice bucket
• 37°C incubator
• Hoechst 33258 in PBS (1 µM)
• D-luciferin dissolved in PBS (100 mg/kg of body weight)
• needles and syringes
• micro-centrifuge tubes for blood samples
Procedure for measuring susceptibility to Plasmodium berghei malaria infection
I. Microchip placement and measurement of body temperature
a. Before and during microchip implantation procedure, mice are lightly anesthetized with isoflurane to minimize any undue movement.
b. The microchip is placed under the skin, between the scapular bones, with a 16-gauge needle placement device.
c. The Bio Medic Data Systems (BMDS) implantable microchip system is then used to identify each mouse and obtain body temperature.
d. With the use of the BMDS hand-held wand, body temperature is collected from each mouse while it is in its own cage, without any need for physical restraint.II. Infection and parasites
a. The Plasmodium berghei ANKA strain PbGFPâLUCSCH is used for all infections.
b. Mice are infected intraperitoneally (i.p.) with 1x106-parasitized red blood cells obtained from a donor mouse.III. Assessment of parasitemia
a. Blood is obtained from the very tip of the tail by clipping it with a clean razor.
b. Approximately 5 µL of whole blood is then fixed in 1 mL of 0.25% glutaraldehyde and stored at 4°C before being stained.
c. Fixed blood is incubated for 1 h at 37°C in the dark with 1 µM Hoechst 33258 in PBS, and samples are analyzed by cytofluorometry.
d. A 424DF44 filter is selected as the emission filter for the blue Hoechst fluorescence.
e. Data are recorded and analyzed by Cellquest 3.2 software.
f. Erythrocytes are gated by forward and side scatter and infected erythrocytes are counted by Hoechst fluorescence.
g. For each sample, 10,000 events are acquired and recorded.
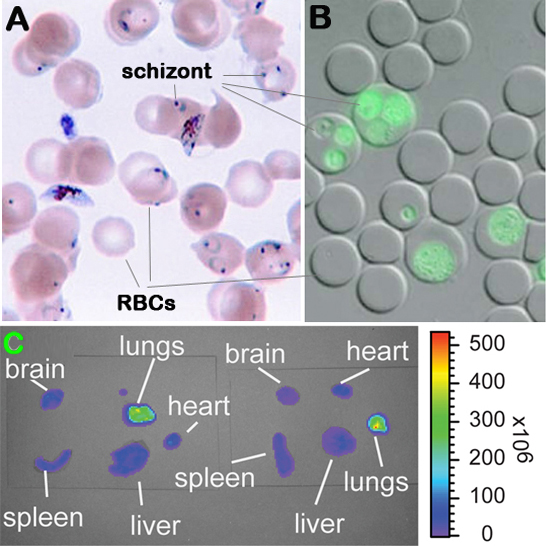
Figure 1. Malaria parasite (Plasmodium berghei) infected erythrocytes and detection of parasitemia and bioluminescence. Panel A: Giemsa stained blood smear. Panel B: Flourescent microscopic image of infected erythrocytes. Panel C: Visualization of luciferase activity in individual organs.
IV. Visualization and quantification of luciferase activity
a. Infected moribund mice are anesthetized by isoflurane gas inhalation before administering D-luciferin intraperitoneally.
b. 2 to 4 min after injection with D-luciferin, mice are euthanized and the organs dissected without perfusion and placed in a Petri dish for imaging.
c. Luciferase activity in dissected organs is then visualized and recorded with intensified-chargecoupled device (I-CCD) photon counting video camera of the in vivo imaging system (IVIS), with a 10 cm field of view (FOV), a medium binning factor, and exposure times between 10â60 s. Rainbow images show the relative level of luciferase activity ranging from low (blue), to medium (green), to high (yellow, red) (see Figure 1C above).
d. Measurements are done using fixed time and region-of-interest settings with the programs LIVING IMAGE (Xenogen) and IGOR-PRO.
e. Relative photon counts of each intact organs are calculated as a percentage of the total photon counts of all organs examined.
Data collected by investigator
• Number parasite infected erythrocytes in blood
• Number of days mice survived parasite infection
• Final body temperature
• Luciferase activity in brain, spleen, lungs, and liver
References
Watanabe M, Yamamoto T, Kakuhata R, Okada N, Kajimoto K, Yamazaki N, Kataoka M, Baba Y, Tamaki T, Shinohara Y. Synchronized changes in transcript levels of genes activating cold exposure-induced thermogenesis in brown adipose tissue of experimental animals. Biochim Biophys Acta. 2008 Jan;1777(1):104-12. Epub 2007 Nov 6.
PubMed 18036333 Winzeler EA. Applied systems biology and malaria. Nat Rev Microbiol. 2006 Feb;4(2):145-51.
PubMed 16362033 Winzeler EA. Malaria research in the post-genomic era. Nature. 2008 Oct 9;455(7214):751-6.
PubMed 18843360 FullText