Ackert1 project protocol
Aging study: Bone mineral density and body composition of 32 inbred strains of mice (2008)
Ackert-Bicknell C, Beamer WG, Rosen CJ, Sundberg JPWith: Juan R, Godfrey D, Coombs HF
Project protocol — Contents
Workflow and sampling
Equipment
Reagents, supplies, and solutions
Procedure: Dual-energy X-ray absorptiometry (DXA)
Data
ReferencesWorkflow
- Ohaus Portable Navigator Series Electronic Toploading Balance (Model NV-210) for measuring body weights
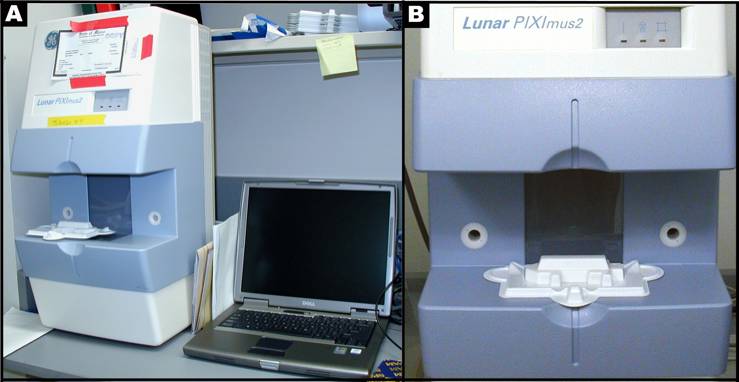
- Lunar PIXImus II Mouse Densitometer (GE Medical Systems Model 51045; Madison, WI, USA), designated computer, and printer.
- Disposable specimen trays with sticky immobilizing tape: Lunar PIXImus Corporation Headquarters, 726 Heartland Trail, Madison, WI 53717.
- Short acting anesthetic: Avertin (Tribromoethanol; 0.2 mL/gm body weight, injected i.p)
Figure 1 A: Lunar PIXImus2 densitometer with integrated PC computer. Figure1 B: Close-up detail of the Lunar PIXImus2 densitometer with specimen tray.Disinfectant and cleaning solutions
Procedure: Dual-energy X-ray absorptiometry (DXA)
Investigator's notes: 20-mo old mice destined for necropsy (Sundberg1) are euthanized using CO2 gas asphyxiation before they are scanned.
I. Preparation for densitometry and collecting image scans
PIXImus (small animal DXA system, PIXImus™, Fitchburg, WI) scanning of mice for BMC and % fat is both accurate and precise although body size must be considered when comparing inbred strains. Full body scans are obtained and X-ray absorptiometry data gathered and processed with manufacturer supplied software (version 1.43.036.008).
a. Before the system is used the PIXImus is calibrated daily with a "phantom mouse" according to manufacturer's protocol.
b. To obtain the PIXImus scans, 6, 12 and 20-mo old mice are weighed and body weight recorded, and then the mice are anesthetized under approved protocols with Avertin (Tribromoethanol; 0.2 mL/g body weight, injected i.p.).
c. Anesthetized mice are positioned dorso-ventral with the tail positioned away or alongside from the body, the front legs extended to the side, and the neck and spine are gently straightened.
d. Then each mouse is placed on the specimen sticky tray (body must be within blue line on the tray) under the PIXImus beam path (see Figure 1B above). Care is taken to ensure that the limbs are extended (i.e. limb bones are not over lapping with each other or with the rib cage) and that with the exception of the head, all of the mouse, including the tail can be viewed in the resulting image.
e. Trays are positioned such that the area of the head is always oriented toward the left from the investigator's point of view, and the mice are positioned dorso-ventral in order to scan the entire body and tail. The X-ray process to obtain a single full scan is approximately 4 min.
f. Disposable plastic trays, with sticky tape for immobilizing mice, can be saved and re-used after cleaning and disinfecting.
II. Measurement acquisition and image scan analysis
Based on PIXImus validation studies (Nagy, 2000; Johnston, 2005) DXA estimated measurements of fat tissue correlate well with measurements obtained from chemical extraction. This is made possible by developing software versions with equations that adequately correct raw DXA measurements.
a. Following the completion of an image scan the DXA system automatically implements specialized software to identify bone tissue from either fat tissue or from lean tissue based on the resulting X-ray densities at two distinct energy levels (Pietrobelli, 1996; Johnston, 2005).
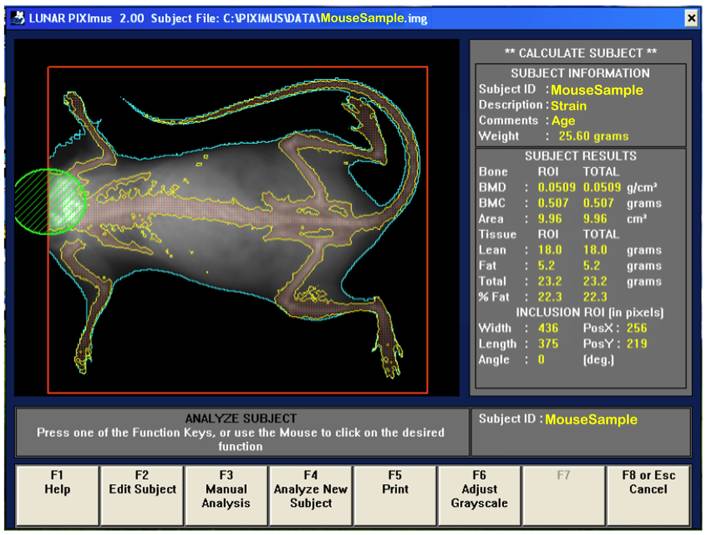
b. Following the completion of a scan, the mouse sample is then outlined in blue and the bones in yellow (see image below).
Investigator's note: Any mouse for which all 4 limbs cannot be seen independently or for which part of the mouse is outside of the detector viewing area was either rescanned or the data for that mouse was removed from the data set. Only whole body data is collected for this data set; no sub-regions of interest.
c. The area to be analyzed is defined by a red box, and area to be excluded from the calculations is defined by a green circle (i.e. excluding the head). The arrow keys are used to adjust to the desired size, in addition to holding the control key down to enlarge or elongate the circle or square areas.
d. Once the head has been excluded, the Enter key is clicked and resulting data measurement is displayed. By pressing F5 a hard copy of the image and the scan analysis result is printed.
e. To prompt the computer to finish the session, F8 or Esc key is clicked once, and then clicked again to return to the main menu screen where the next subject to be tested begin.
f. Acquired data is saved on the hard drive and CD disk for later archiving.
Safety
For safety, gloves must be worn and radiation safety guidelines are strictly adhered to, such that technicians must be 6 feet away from the PIXImus machine during scanning.
Data collected by investigator
PIXImus data are from the whole body exclusive of the head. Areal bone mineral density (aBMD), bone mineral content (BMC), bone area, total tissue mass, total tissue area, lean tissue mass, percent body fat, body weight, and body length were measured.
Bone area measurement is generated by outlining or specifying the limits or dimensions of the entire skeletal bone regions of the body (limbs, neck, spine, and tail), excluding the head, following a full body X-ray scan.
Bone mineral content (BMC) is generated from PIXImus density scans which are assessed for accuracy using a set of 0.0 mg to 2,000 mg of hydroxyapatite standards. According to the DXA system, bone mineral content (measured as the attenuation of the X-ray by the bones being scanned) is divided by the area (also measured by the machine) of the site being scanned to obtain bone mineral density (BMD).
BMC = Bone mineral content (g)
B-area = Bone area (cm2)
BMD = Bone mineral density = BMC ÷ B-area (g/cm2)Fat tissue mass = all tissues with low density (x-ray scan)
T-area = Total body area (cm2)
% Fat = (Fat tissue mass ÷ Total body tissue mass ) x 100
TTM = Total Tissue Mass (g)
LTM = Lean Tissue Mass (g)
BMI = body mass index [kg/m2] = (body weight in g x (kg/1000g)) ÷ ((body length in cm x (m/100cm)) x (body length in cm x (m/100cm))
References
Ackert-Bicknell CL, Karasik D, Li Q, Smith RV, Hsu YH, Churchill GA, Paigen BJ, Tsaih SW. Mouse BMD quantitative trait loci show improved concordance with human genome-wide association loci when recalculated on a new, common mouse genetic map. J Bone Miner Res. 2010 Aug;25(8):1808-20.
PubMed 20200990 Beamer WG, Shultz KL, Ackert-Bicknell CL, Horton LG, Delahunty KM, Coombs HF 3rd, Donahue LR, Canalis E, Rosen CJ. Genetic dissection of mouse distal chromosome 1 reveals three linked BMD QTLs with sex-dependent regulation of bone phenotypes. J Bone Miner Res. 2007 Aug;22(8):1187-96.
PubMed 17451375 Beamer WG, Shultz KL, Coombs HF 3rd, DeMambro VE, Reinholdt LG, Ackert-Bicknell CL, Canalis E, Rosen CJ, Donahue LR. BMD regulation on mouse distal chromosome 1, candidate genes, and response to ovariectomy or dietary fat. J Bone Miner Res. 2011 Jan;26(1):88-99.
PubMed 20687154 Nagy TR, Clair AL. Precision and accuracy of dual-energy X-ray absorptiometry for determining in vivo body composition of mice. Obes Res. 2000 Aug;8(5):392-8.
PubMed 10968731 Pietrobelli A, Formica C, Wang Z, Heymsfield SB. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol. 1996 Dec;271(6 Pt 1):E941-51.
PubMed 8997211 Pietrobelli A, Wang Z, Formica C, Heymsfield SB. Dual-energy X-ray absorptiometry: fat estimation errors due to variation in soft tissue hydration. Am J Physiol. 1998 May;274(5 Pt 1):E808-16.
PubMed 9612238 Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19(2):109-24.
PubMed 19392647 FullTextWergedal JE, Sheng MH, Ackert-Bicknell CL, Beamer WG, Baylink DJ. Genetic variation in femur extrinsic strength in 29 different inbred strains of mice is dependent on variations in femur cross-sectional geometry and bone density. Bone. 2005 Jan;36(1):111-22. Epub 2004 Nov 24.
PubMed 15664009 Wergedal JE, Sheng MH, Ackert-Bicknell CL, Beamer WG, Baylink DJ. Mouse genetic model for bone strength and size phenotypes: NZB/B1NJ and RF/J inbred strains. Bone. 2002 Dec;31(6):670-4.
PubMed 12531560