HMDPpheno3 project protocol
Heart rate and blood pressure in males of 58 strains of mice (2012)
Korshunov VAWith: Smolock EM, Ilyushkina IA, Ghazalpour A, Gerloff J, Murashev AN, Lusis AJ
Project protocol — Contents
Workflow and sampling
Workflow and sampling
Equipment and supplies
Procedures
Definitions and calculations
Data
ReferencesWorkflow
Mice acclimated and trained to undergo blood pressure measurement for 5 days Last 2 days of training used for measurements System cleaned and readied after each batch of mice
- Visitech system, Cary, NC
- Thermometers: alcohol or mercury filled
- Bandage scissors
- Balloons (for tail cuffs)
- Medical tape
- Paper towels
- Smooth tubing clamps
- Heat gun
cleaning and disinfecting solutions
Acclimation to test conditions
Mice are acclimated and trained to test conditions for 3 days prior to obtaining recorded measurements.
Procedure: Blood pressure and pulse analysis using the Visitech System
Measurements of systolic blood pressure and pulse rate are obtained from unanesthetized mice with minimal restraint using the Visitech system. The Visitech system is a non-invasive blood pressure and pulse analyzer that can accommodate 4 mice simultaneously per session. In order to obtain consistency in the results, mice are first conditioned in the system the same time everyday for at least 3 days.
Essential checklist
a. Specimen platforms are set at exactly at 38°C for maintaining core body temperature during testing (VERY IMPORTANT). After enough time is allowed for temperature to stabilize, the correct temperature should register when measured in the middle of the heating platform. Failure to do so, not only compromises the results, but also may be fatal to mice in cases of overheating (especially small mice).
b. Long 5-6" balloons are cut into 4 equal sections of 1 1/3" pieces. These balloon pieces are then installed and threaded through the tail-cuffs. Without stretching the balloons, they are shrunk and customized to fit the tail-cuffs with the use of a heat gun or a lighter.
c. The balloons are checked for holes by pumping air into the tail-cuffs at pressures not greater than 200 mm Hg that remain stable over a given time. In the event that the observed pressure is steadily lost, air is pumped up again and the balloon with the hole is systematically identified using a pair of smooth tubing clamps (not hemostats or forceps as they may damage the tubing) and then replaced.
d. Pressure is calibrated according to manufacturer's protocol. Briefly, by connecting directly the air supply tube to the sphygmomanometer, and inputting the values observed in the attached mercury column, these pressure calibrations are then compared with those displayed on the computer following inflations of the tail-cuff balloons at appropriate pressures.
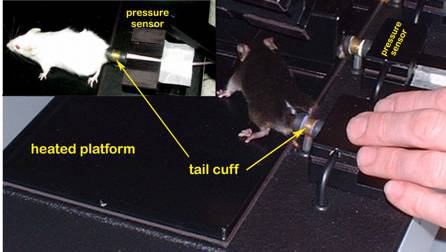
Figure 1. The Visitech System for blood pressure and pulse measurements in mice.
Figure 2. Mice positioned for blood pressure measurement using tail-cuff method.Obtaining pulse and blood pressure measurements
a. Fully acclimated and trained mice are picked up by their tails and gently placed on a temperature-stabilized (38°C) platform. At least two thermometers are taped in the middle and on the side of the platform and covered with magnetic restrainers to provide additional means to monitor and to assure proper platform temperature.
b. The mice are then restrained in place (without anesthesia) using magnetized holders. Care is taken to prevent the feet from getting pinched by the magnets.
c. To facilitate measurement efficiency, automated 4-stage sphygmomanometers for mice are used to test 4 mice simultaneously in a given session.
d. Once the mice are properly secured, their tails are then passed through the cuffs and the optical hemodrometers, and the exposed proximal tail segments are fixed in place with bandage tape.
e. The mice may be left in the optical position for 5-10 min to promote body temperature stabilization. Any evidence of mouse sweating during measurement is noted, for it is likely indication of non-optimal or unregulated temperature.
f. The tail-cuff system is then turned ON to begin the process; air is automatically pumped into the balloons, consequently applying pressure on the tail vessels, so that waveforms of blood vessel expansions and contractions can be optically sensed and recorded.
g. Simultaneous recording of systolic blood pressure, diastolic blood pressure, and pulse rate measurements are automatically recorded multiple times session.
h. In order to obtain reliable and accurate representation of blood pressure and pulse rate, measurements are taken the same way and at the same time for the last two days.
i. After all data are collected and saved, the bandage tape is gently removed from the tail; mice are allowed to come out of the restraint on their own and are returned to their respective cages.
j. At the end of each session the platforms are cleaned.Diastolic blood pressure: blood pressure during peak cardiac distension and relaxation.
Systolic blood pressure: blood pressure during peak cardiac contraction.
Tail pulse: throbbing of tail arteries as a consequence of heartbeat.
Sphygmomanometer: blood pressure metering device.
Hybrid mouse diversity panel (HMDP): consists of a population of over 100 inbred mouse strains selected for usage in systematic genetic analyses of complex traits; 29 classic parental inbred and panels of recombinant inbred (RI) mice, including the BXD, CXB, BXA/AXB, and BXH panels.
Data collected by investigator
- systolic blood pressure
- pulse rate