Chang2 project protocol
Morphological examination of the eye in C57BL/6J-Chr#PWD/Ph/ForeJ mouse chromosome substitution strains, age 6-9 months (2012)
Chang B, Donahue LProject protocol — Contents
Workflow and sampling
Equipment and supplies
Reagents and solutions
Procedure: Evaluation of eye morphology
Definitions
Data
References
Workflow and samplingWorkflow
Mice examined grossly for overall health and appearance Anterior segment of the eye assessed Eyes dilated with 1% atropine sulfate Lens checked for transparency and/or cataracts Posterior segment of the eye assessed Slit lamp biomicroscope: for the examination of the anterior chamber of the eye (Nikon, Tokyo, Japan)
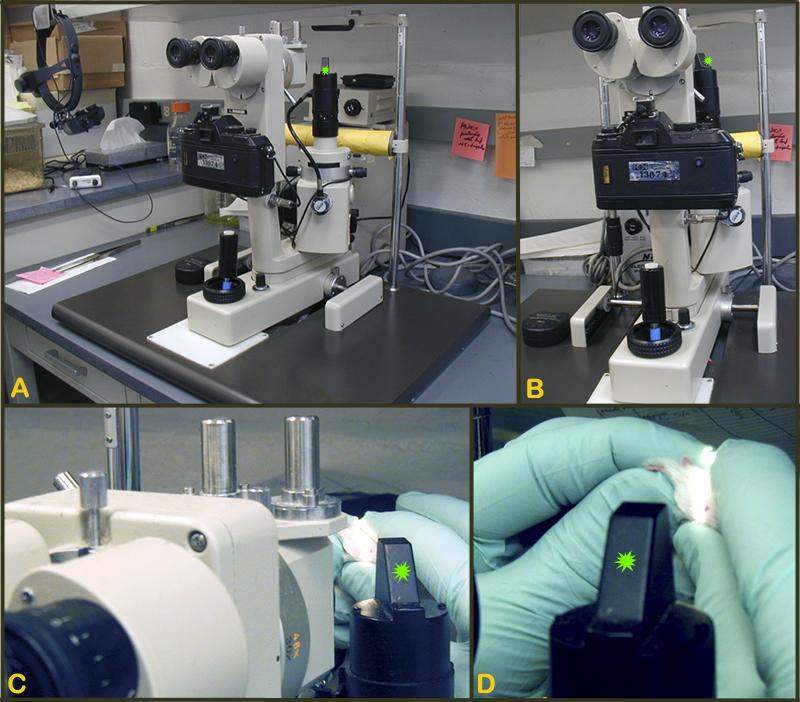
Figure 1. A-B: Slit lamp biomicroscope for the examination of the anterior segment (i.e. iris, cornea, lens) of the eye. C-D: Close-up of the light source (green asterisk) of slit-lamp in A-B.Indirect ophthalmoscope (HEINE OMEGA, Germany) for the examination of the posterior chamber or fundus of the eye (American Optical, Rochester, NY, USA)
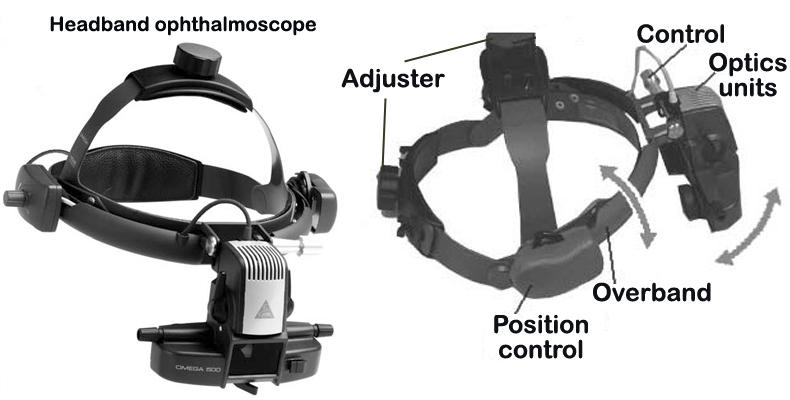
Figure 2: Indirect ophthalmoscope.
- Diopter (D) condensing lens: 90D or 60D and 78D superfield lenses (Mentor, OH, USA)
- Camera: to photograph the fundus of the eye (Nikon, Kowa RV-2, or Kowa Genesis; Tokyo, Japan)
- Film: Kodak Elite 200 ASA slide film
- Atropine sulfate 1%: for pupillary dilation
- 1% cyclopentolate: for pupillary dilation
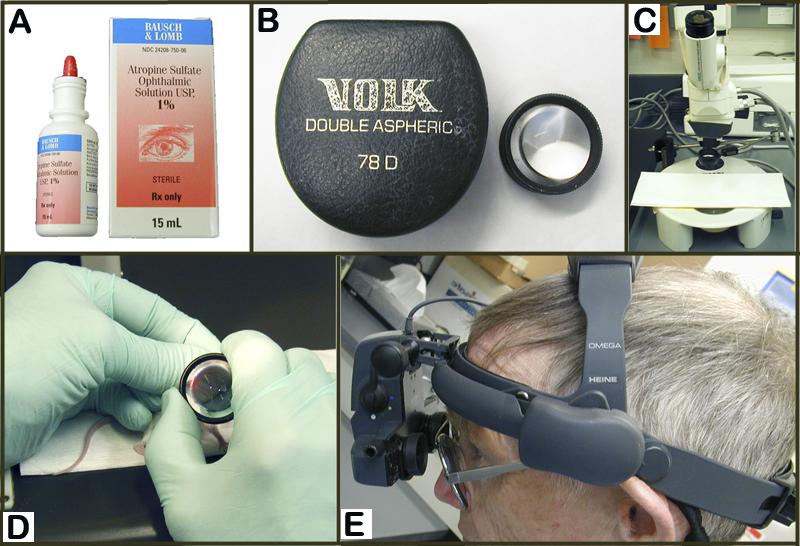
Figure 3. A: 1% Atropine topical drop preparation for dilating the eye. B: 78D diopter lens used in conjunction with indirect ophthalmoscope as shown in D and E. C: Camera-scope for photographing the fundus of the eye. D: handling of the diopter lens during eye examination. E: fitting of the indirect ophthalmoscope for funduscopic inspection.Acclimation to test conditions
In general all mice are acclimated in the mouse rooms where eye examinations are conducted.
Procedure: Evaluation of eye morphology
a. Visual inspections of the eyelids, globe, cornea, sclera, and iris are performed.
b. The cornea is examined for clarity, size (buphthalmos vs. microcornea), surface texture, and vascularization using a slit lamp (see Fig. 1, Fig. 4).
c. The iris is checked for pupilary size and central location, iris constriction when exposed to bright light, reflected luminescence, and synechia (adhesion to the cornea or lens).
d. The eye is then dilated with 1% atropine and the lens is checked for cataracts using the slit lamp.
e. Once the eye is dilated, an indirect ophthalmoscope and a hand-held diopter lens are used (Fig. 2 and Fig. 3) to examine the fundus for signs of retinal degeneration (such as retinal vessel attenuation or retinal pigment epithelial (RPE) disturbances), as well as for drusen or deposits or optic cup (nervehead) abnormalities (Fig. 4 to Fig. 7).
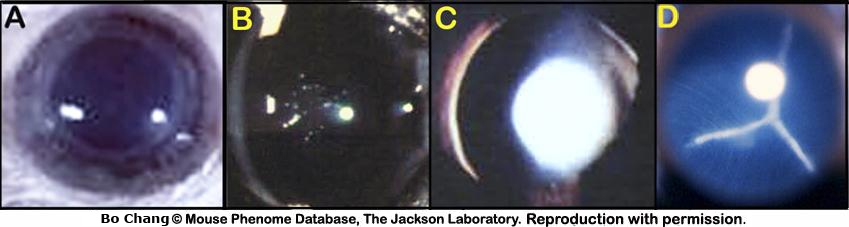
f. Fundus photographs are then taken.Cornea: Morphological alterations of the cornea include, but are not limited to, changes in the normal transparency of the cornea (i.e. cloudy cornea, white cornea/corneal opacity), presence of corneal scarring (i.e. corneal spot) or fibrosis and neovascularization (i.e. corneal vessels), and signs of corneal trauma (i.e. corneal tear). Severity can be described as diffuse or focal (small, medium, and/or large spot). See examples in Fig. 4.
corneal (color) opacity: alterations in the normal transparency of the cornea (i.e. diffusely hazy, cloudy or white, focal small-large spot) may be congenital, infectious, traumatic, or idiopathic in origin (see image examples below).
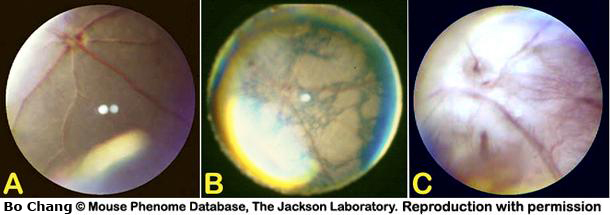
Lens: Morphological alterations of the lens include, but are not limited to, cataract formations originating from the lens nucleus or lens sutures, aberrant adhesions of the lens to the cornea (i.e. corneal-lens synechia), lens location, and alteration of the overall transparency of the lens fibers (i.e. lens haze, lens snowflakes, lens spot, see examples in Fig. 5).
lens cataract: morphologically diffuse or focal alterations in the crystalline lens (i.e. nuclear cataract, suture cataract, snowflake cataract) of the eye or its capsule, potentially resulting in impaired vision, most likely congenital, or idiopathic in origin (Fig. 5).
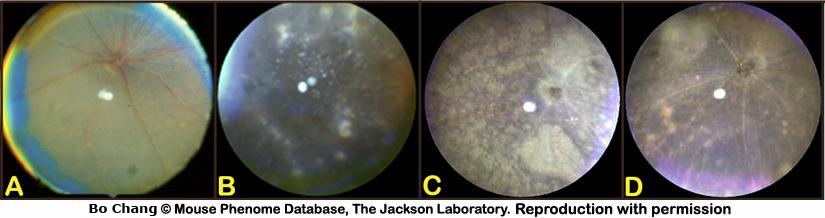
Retina: Morphological alterations of the retina include, but are not limited to, changes to the retinal pigment epithelium (RPE, areas of pigment loss, depigmented spots, mottled), changes in the size of the retinal vessels (i.e. poor and thin retinal vessels), appearance of the the optic disc and nerve (i.e. retinal coloboma, enlarged optic cup), and severe degeneration of the retinal layer (i.e. rd mutations, see examples in Fig. 6 and Fig. 7).
- retinal degeneration: progressive loss of retinal layers (i.e. photoreceptor rods and/or cones layer, ganglion cell layer) with concomitant secondary retinal changes (i.e. marked changes in retinal pigmentation, attenuation of retinal vessels, changes in appearance and size of the optic cup), most likely congenital or idiopathic in origin (see example images below).
- retina incidental findings: strain-specific, non-specific, or secondary changes (i.e. retinal vessel attenuation) in the retina, may be congenital (i.e. retinal optic cup anomalies, retinal coloboma), infectious, traumatic, or idiopathic in origin (i.e. retinal detachment).
- alterations in retinal pigmentation: strain-specific variations in retinal pigmentation, may be a component of progressive retinal degeneration, incidental changes in the pigment epithelium (RPE) of the eye, or part of a developmental dysgenesis (see example in Fig. 7). At least some of the same genetic components for retinal pigmentation likely affect coat color, as these two phenotypes are highly correlated.
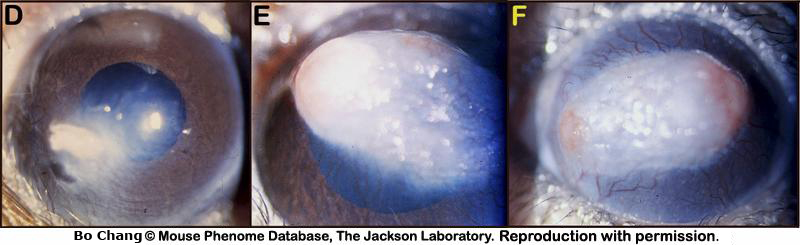
Figure 4. A: Superficial gross appearance of the normal eye and cornea. B and C: Superficial gross appearance of a bilateral corneal change in transparency. D: Small spot with peripheral cloudy appearance of the cornea. E: Large white spot with signs of neovascularization in the cornea. F: Diffusely white cornea with neovascularization and two foci of corneal ulcerations.
Figure 5. A: Fully dilated eye showing normal, clear and transparent, lens. B: An example of lens snowflake cataract. C. An example of a lens nuclear cataract. D: An example of a lens suture cataract.
Figure 6. A: Appearance of a normal funduscopic examination of the retina. B and C: Examples of advanced retinal degeneration in a pigmented mouse (B) and an albino mouse (C). Note the pronounced mottling of the retina and the attenuation of the retinal vessels compared to the normal eye.
Figure 7. A: Normal funduscopic appearance of the retina. B: Example of retinal (punctate hypopigmentation) spots. C: Example of large areas of retinal hypopigmentation (spot). D: Example of moderate areas of hypopigmentation (spots) with signs of mottling. Note the attenuation of the retinal vessels compared to the normal retina in A.MPD Notes (severe, highly penetrant phenotypes)
Summary list of strains with eye phenotypes
Iris dysgenesis phenotype (bilateral): PL/J exhibits iris synechia and atrophy with iris threads or tissue in the pupil, and the genetic basis of these phenotypes are currently under intense investigation.
Iris transillumination defect (bilateral): Brown coat colored DBA/2J, and black coat colored SEA/GnJ already exhibit this defect in both sexes at 6-7 wks-of age.
Opaque cornea: C3H/HeJ exhibits an opaque corneal phenotype described as focal white spots of variable size. This characteristic may be sex-linked as females show a higher incidence over males.
Cataracts: CBA/J exhibits bilateral cataracts described as "hazy" according to the definition by West and Fisher, 1985); C57BL/6J exhibits bilateral cataracts described as "snowflake"; RIIIS/J exhibits bilateral cataracts that are so severe that they prevent normal funduscopic examination of the eyes (unable to characterize retina); SEA/GnJ exhibits bilateral suture cataracts.
Retinal degeneration (bilateral): By 6-7 wks of age the following strains already show a congenital retinal degeneration phenotype: BUB/BnJ, C3H/HeJ, CBA/J, FVB/NJ, JF1/Ms, MOLF/EiJ, NON/ShiLtJ, PL/J, SJL/J, SWR/J. Retinal degeneration in NON/ShiLtJ is particularly severe with attenuated retinal vessels.
Retinal coloboma (bilateral): In addition to retinal degeneration, JF1/Ms exhibits a mottled retina and retinal coloboma.
Retinal hypopigmentation (bilateral): LP/J and PWK/PhJ exhibit this phenotype (LP/J is sex-independent; PWK/PhJ males exhibit hypopigmentation, while in females there is a lower incidence).
Large optic cup (bilateral): SEA/GnJ mice have an abnormally large optic cup. The incidence was higher in males than females (100% vs. 78%, respectively).
Data collected by investigator
Gross morphological changes in the eyes:
- retinal spots (multifocal circumscribed areas of hypopigmentation)
- eyelessness
- corneal opacity
- punctate cataract