Mills1 project protocol
Aging study: Chromosome instability and apoptosis in 30 inbred strains of mice (2008)
Mills KWith: Schultz D, Godfrey D, Alley T
Project protocol — Contents
Workflow and sampling
Equipment
Reagents, supplies, and solutions
Procedure for measuring micronuclei in peripheral blood
Procedure for measuring apoptotic splenocytes
Definitions and formulas
Data
References
Workflow
Peripheral blood collected and processed Spleen harvested and processed Annexin-V assay (apoptosis) on splenocytes (flow cytometry) viable, dead, early, and late apoptotic splenocytes Micronuclei assay (DNA damage) on non-nucleated peripheral blood cells (flow cytometry) non-nucleated blood cells with and without micronuclei
- Micro-pipetters
- Small benchtop microcentrifuge (Eppendorf 5415C) (see image panel A below)
- Large refrigerated centrifuge (Beckman Coulter Avanti J-E, JS 5.3 rotor), see image panels B to D below.
Figure 1. Refrigerated centrifuge.- FlowJo V.7, software for data acquisition/analysis (TreeStar, Inc., Ashland, OR)
- Flow cytometer: FACSCalibur (BD Pharmingen, San Jose, CA) see Figures below.
Figure 2A: FACSCalibur setup. Figure 2B: Front view of the FACSCalibur setup. Figure 2C: FACSCalibur sample port.Reagents, supplies, solutions Â
⢠1.5 mL Eppendorf microcentrifuge tube
⢠Conical tubes, 15 mL polypropylene
I- Micronuclei measurement essentials
⢠Heparin solution (Sigma H3149) dilute to 500 USP units heparin/mL in PBS
⢠0.9% buffered saline solution (0.9% sodium chloride, 5.3 mM sodium bicarbonate):
=> 18.0 g NaCl
=> 0.9 g sodium bicarbonate
=> Bring up to 2.0 L with H2O
=> pH to 7.3
⢠Propidium iodide (PI) solution (1.25 µg/mL) (Sigma 287075) => diluted with 9% buffered saline
⢠CD71/FITC (Invitrogen RM5301)
⢠RNAse A (10 mg/mL)(Sigma R5250) => diluted with 9% buffered saline
⢠Staining solution:
=> 79 µL 9% buffered saline
=> 10 µL RNAse A
=> 1 µL CD71/FITC antibody solution
⢠Methanol
II- Apoptotic measurement essentials
⢠Annexin-V: FITC Apoptosis Detection Kit (BD Pharmingen 556547)
=> 10X Annexin-V binding buffer (made fresh as 15 mL of 1X buffer with H2O)
=> FITC Annexin-V conjugated antibody
=> Propidium iodide (PI) staining solution
⢠Phosphate buffered saline (PBS), kept at 4°C (Invitrogen 14190-235)
⢠Hemocytometer counting chamber, Reichert Bright-Line (Fisher 02-671-5)
⢠Surgical scissors and forceps

⢠Cell strainer, BD Falcon (Fisher 08-771-19)
⢠Glass rod (Fisher 11-380B)
⢠Tissue culture dish, 6 cm treated
⢠Round bottom tube, BD Falcon (BD Pharmingen 352052)Procedure for measuring micronuclei in non-nucleated peripheral blood cells
I. Blood collection
Blood samples are collected from retro-orbital plexus using micro-capillary tubes. Minimum required blood is 270 µL (about 12 drops, plus one capillary tube).II. Cell preparation
a. At least 1 day before blood samples are collected, 15 mL polypropylene conical tubes (one tube per blood sample) are prepared by adding 2 mL methanol and storing at -80°C.
b. Using a pipette, 50 µL of blood is drawn and transferred to a 1.5 mL microcentrifuge tube containing 250 µL of heparin solution kept on ice.
c. Blood samples are centrifuged at 14,000 rpm for 5 min at 4°C to separate cells from plasma.
d. Top plasma layer is removed and stored at -80°C.
e. Cell pellet is resuspended in 200 µL FACS buffer and transferred to 12 x 75 mm polypropylene tube.
f. Cell suspension (180 µL) is delivered forcefully into a conical tube containing cold methanol.
g. To break up any aggregates, sample tubes are finger-flicked several times and immediately placed back into the -80°C freezer for at least 24 h.Investigator's note: When pipetting into cold methanol, do not allow pipette tip to touch side of tube or methanol. Whole blood cells can be stored in cold methanol indefinitely.
III. Labeling cells
a. Sample tubes are retrieved from the -80°C freezer and finger-flicked several times to break up cellular aggregates.
b. Immediately ice-cold 9% buffered saline (12 mL) is added to the cells and mixed by gently inverting the tube, return to ice.
c. Samples are centrifuge at 600 x g for 5 min at 4°C.
d. Supernatant is aspirated and discarded with P200 pipette tip and cells are resuspended in residual volume.
e. Resuspended blood cells (10 µL) are transferred to FACS tube containing 90 µL of staining solution* and mixed well.
f. Samples are protected from light and incubated for 30 min on ice and then 30 min at room temperature.
g. Cold propidium iodide solution (1 mL) is added. Samples kept on ice till analyzed.* Antibodies are titrated to determine optimal staining levels with minimum background staining.
IV. Flow cytometry
The BD FACSCalibur (see Figure 2 A-C above) must be compensated to project a good dot plot (following manufacturer's protocol). [FL1 channel (CD71-FITC) x FL3 channel (PI)]. At least 500,000 live events are collected for each sample.V. Analysis of non-nucleated blood cells using FlowJo v.7 software
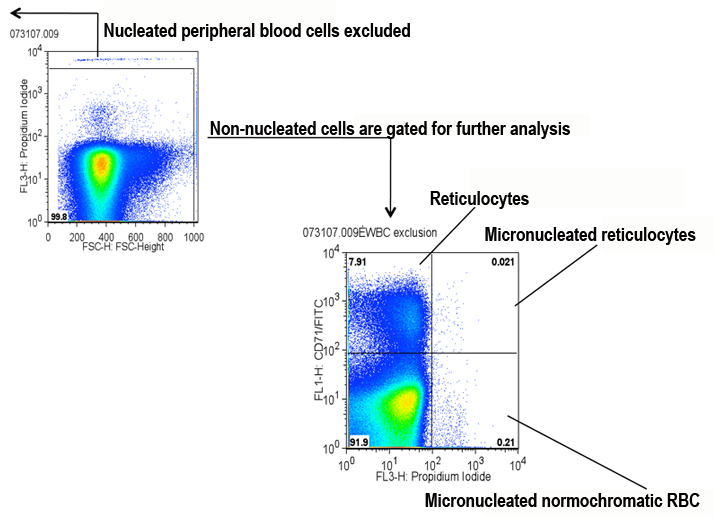
a. Nucleated cells (PI high) are excluded, and non-nucleated cells are gated for analysis via the FL3 channel x FSC-height channel (see Figure below).
b. A 4-quadrant gate is set in the two-parameter plot to define all four populations of non-nucleated peripheral blood cells (see Figure below).Quadrants:
1. PI+ CD71+ (micronucleated reticulocytes)
2. PI- CD71+ (reticulocytes without micronuclei)
3. PI- CD71- (normochromatic red blood cells without micronuclei)
4. PI+ CD71- (micronucleated normochromatic red blood cells)
Submitting PI notes: Propidium iodide (PI) binds to DNA and RNA. RNA is destroyed by RNAse A in the staining solution, leaving DNA intact. Nucleated cells are excluded in this procedure, revealing micronuclei in the remaining non-nucleated cells.
Procedure for measuring apoptotic splenocytes
I. Harvesting spleen and isolating splenocytes
a. Aged mice destined for necropsy are processed.
b. Approximately 20 mg of the spleen is harvested at a standard location using clean sharp surgical scissors and forceps, and is placed immediately in a tissue culture dish containing 5 mL cold phosphate buffered saline (PBS) as other samples are being harvested.
c. Sectioned spleen is placed into the cup portion of a cell strainer and then transferred back into the culture dish.
e. Using forceps and scissors, the spleen is further sectioned into 5-6 pieces.
f. Using a glass rod, the pieces are gently mashed and pushed through the cell strainer.
g. The cell strainer is removed from the culture dish and the splenocyte suspension is pipetted into a 15 mL conical tube.
h. The splenocyte suspension is then centrifuged at 1000 rpm at 4°C for 5 min to form a cell pellet.
i. Following centrifugation, the supernatant is aspirated and discarded, and the pelleted cells resuspended in 1 mL cold PBS.
j. Concentration of splenocytes in the suspension is obtained using a hemocytometer counting chamber.II. Splenocyte preparation and labeling
a. After measuring the concentration of splenocytes in the 1 mL suspension, a calculated volume containing 1 x106 cells is transferred into a 1.5 mL microcentrifuge tube containing 1 mL cold PBS.
b. The sample is centrifuged at 1,500 rcf (4,000 rpm) for 4 min.
c. The supernatant is aspirated and discarded, and the pelleted cells are resuspended in 1 mL cold PBS for subsequent washing.
d. The sample is again centrifuged at 1,500 rcf (4,000 rpm) for 4 min.
e. The supernatant is aspirated and discarded, and the pelleted cells are resuspended in 1 mL 1X Annexin-V binding buffer.
f. Splenocyte suspension (100 µL) is transferred in to a FACS tube.
g. To the 100 µL of splenocyte suspension, 5 µL Annexin-V-FITC antibody and 5 µL propidium iodide (PI) labeling solutions are added.
h. Sample tube is gently mixed and incubated for 15 min in the dark at room temperature.
i. Binding buffer (400 µL of 1X) is added and flow cytometry is performed within 1 h.
III. Flow cytometry
The BD FACSCalibur (see Figure A to C above) must be compensated to project a good dot plot (following manufacturer's protocol). [FL1 channel (Annexin-V FITC) x FL3 channel (PI)]. At least 20,000 live events are collected for each sample.
IV. Analysis of apoptotic splenocytes using FlowJo v.7
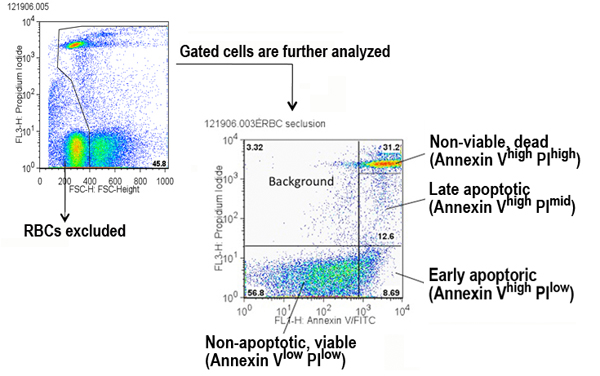
a. Red blood cells (PI high) are excluded, and remaining cells are gated for analysis via the FL3 channel x FSC-height channel (see Figure below).
b. Gates are set in the two-parameter plot to define four populations of splenocytes (see Figure below).
Areas:
1. Annexin-Vlow PIlow (viable cells)
2. Annexin-Vhi PIlow (early apoptosis)
3. Annexin-Vhi PImid (late apoptosis)
4. Annexin-Vhi PIhi (non-viable cells, dead)Note: Additional gating parameters were set to obtain the following populations of splenocytes (these data are available in the Supplemental file only): viable Annexin-V hi; viable Annexin-V mid.
RCF (rcf): relative centrifugal force (gravity x force, or g-force). This is the actual force exerted on the contents of the spinning rotor. RCF is calculated using the following equation:
rcf = 1.1118 x 10-5 x r x rpm2
where r = radius of the rotor
Data collected by investigator
Micronuclei assay (DNA damage): populations listed in the procedure (above) from 6, 12, and 20 month-old mice, including reticulocytes (retic) with and without* micronuclei; normochromatic red blood cells (RBC) with micronuclei. Total micronucleated cells (all PI+ cells) computed by adding values for micronucleated retic and RBC.
Annexin-V assay (apoptosis): populations listed in the procedure (above) from 6, 12, and 20 month-old mice, including viable* and non-viable* splenocytes; early and late apoptotic splenocytes. Total viable apoptotic splenocytes (Annexin-V hi) computed by adding values for early (PI low) and late (PI mid) apoptosis.
References
Shima N, Hartford SA, Duffy T, Wilson LA, Schimenti KJ, Schimenti JC. Phenotype-based identification of mouse chromosome instability mutants. Genetics. 2003 Mar;163(3):1031-40.
PubMed 12663541 FullTextVermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995 Jul 17;184(1):39-51.
PubMed 7622868