Lake2 project protocol
SHIRPA assessment, C57BL/6J-Chr#A/J/NaJ mouse chromosome substitution panel (2005)
Lake J, Donahue L, Davisson MTProject protocol — Contents
Workflow and sampling
Equipment
Reagents, supplies, and solutions
Procedure for screening behavioral and functional profiles using SHIRPA
Data
References
Workflow (the same set of mice were tested in Lake3)
Behavioral and physical assessments Latency to fall Overall grip strength (3 trials) Open arena, latency to perimeter, latency to hole, arena entries, stretched attends, head dunks, hole visits, arena rearings, perimeter rearings, line crossings, grooming episodes, fecal boli, urine puddles SHIRPA Viewing Jar (2L Pyrex beaker, 12.5cm diameter x 19cm height)
Figure 1
SHIRPA Arena: Thoren polycarbonate weaning cage (30.7cm w x 30.7cm L x 14.2cm H)
Figure 2
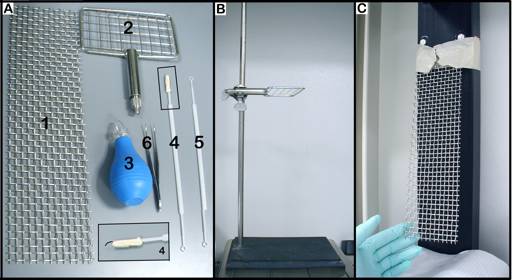
Figure 3. SHIRPA tools. A. 1) vertical woven grid (30 x 8 cm; wire ~0.050" gauge), 2) horizontal woven grid (30 x 8 cm; wire ~0.050" gauge), 3) rubber bulb, 4 & 5) plastic probes, 6) curve tweezers; B. stand with horizontal grid; C. holding board with vertical grid.
- Cleaning supplies: paper towels, 70% alcohol.
- Masking tape: for securing vertical wire-mesh and tail weights
Acclimation to test conditions
Mice are allowed to acclimate to animal room over the weekend.
Procedure for screening behavioral and functional profiles using a modified SHIRPA
• The SHIRPA primary behavioral screen provides a behavioral and functional profile by observational assessment of mice. (SHIRPA was first described by Rogers et al. (1977) and stands for: SmithKline Beecham, Harwell, Imperial College School of Medicine, Royal London Hospital, Phenotype, Assessment.) This screen has been modified and evaluates gait or posture, motor control and coordination, changes in excitability and aggression, salivation, lacrimation, piloerection, defecation, and muscle tone. All parameters are scored to enable comparison of results both over time and between different laboratories.
• Mice are assessed for various behaviors as described below in a view jar, observation arena, horizontal and vertical grids, as well as during tail suspension and supine restraint.
• Set-up View Jar (2L Pyrex beaker), Observation Arena (wean cage), Horizontal and vertical grids.
• All equipment is arranged in the same order for each test.
• The entire test area, including Arena, View Jar, and Grids are wiped down with ethanol and allowed to dry completely before the beginning of each test.
Tests performed in sequential order
a. A mouse is placed in the View Jar for 30s, and observed and scored for body position, spontaneous activity, tremor, twitches, defecation, and urination (see Figure 1, Table 1).
b. The mouse is then removed from View-Jar and placed in the viewing Arena by tipping the jar and allowing the mouse to drop into the wean cage.
c. While transferring, the mouse is evaluated and scored for transfer arousal. In the Arena, the mouse is evaluated for palpebral closing, piloerection, startle response, gait, pelvic elevation, tail elevation, hindlimb splay, touch escape, finger approach, positional passivity, defecation, and urination (see Figure 2, Table 2). A fear assessment is made based on the overall response in the arena. The mouse is then handled sequentially for eliciting a response (called positional passivity) while transferring to the next test.
d. Next, the mouse is suspended via its tail and moved from the Arena to the horizontal wire-grid.
e. While descending toward the grid, the mouse is evaluated and scored for visual placement (see Figure 3A, Table 3).
f. Once the mouse is has on all four limbs on the grid, it is evaluated for body tone, pinna reflex, corneal reflex, withdrawal reflex, and crossed extensor reflex (see Figure 3 A4, Table 3).
g. Subsequently, from a standing position the mouse is held by tail suspension for 15s.
h. While in mid-air the mouse is assessed for hind-limb splay, trunk-curl, and limb-grasp (see Table 4).
i. After being suspended the mouse is lowered via its tail again towards the horizontal wire-grid. Once the mouse has grasped the wire with its forelimbs (only) it is rotated horizontally by the tail.
j. The ability of the mouse to maneuver and to negotiate its position in the horizontal wire platform is scored based on hindlimb response (see Figure 3B, Table 5).
k. Next, the mouse is handled and placed in a supine restraint, along its cervical-thoracic dorsal skin and gently scuffed.
l. While securely restrained in a recumbent position, the mouse is further evaluated for skin color, limb tone, abdominal tone, lacrimation, salivation, biting, irritability, provoked biting, irritability, aggression, and vocalization (see Table 6).
m. The mouse is kept recumbent or in supine restraint for the examination of its righting reflex (see Table 7).
n. Then the mouse is placed on a vertical wire-grid with its head upward, then downward, and evaluated and scored for catalepsy and negative geotaxis (see Figure 3C, Table 8).
o. The mouse is returned to its home-cage.
p. All equipment is cleaned with ethanol and allowed to evaporate completely.Data collected by investigator
Body position Neuromuscular function Activity level Activity & motor function Tremor Activity & motor function Twitches Activity & motor function Defecation (jar) Behavior-anxiety Urination (jar) Behavior-anxiety Transfer arousal Behavior-anxiety Palpebral closing Autonomic function Piloerection Autonomic function Air puff startle reflex Autonomic function Gait Neuromuscular function Pelvic elevation Coat color & appearance Tail elevation Coat color & appearance Fear Behavior-anxiety Hindlimb pattern Coat color & appearance Touch escape Sensorimotor function Finger approach Sensorimotor function Positional passivity Neuromuscular function Defecation (arena) Behavior-anxiety Urination (arena) Behavior-anxiety Visual placing Sensorimotor function Body tone Neuromuscular function Pinna reflex Sensorimotor function Corneal reflex Sensorimotor function Toe pinch Sensorimotor function Crossed extensor reflex Sensorimotor function Suspended hindlimb splay Coat color & appearance Trunk curl Autonomic function Hindlimb grasp Autonomic function Horizontal rotation response Sensorimotor function Skin color Coat color & appearance Hindlimb tone Neuromuscular function Abdominal tone Neuromuscular function Lacrimation Autonomic function Salivation Autonomic function Provoked biting Behavior-wildness Irritability Behavior-wildness Aggression Behavior-wildness Vocalization Behavior-wildness Righting reflex (held/released) Sensorimotor function Righting reflex (flipped) Sensorimotor function Catalepsy Neuromuscular function Negative geotaxis Neuromuscular function
References
Masuya H, Inoue M, Wada Y, Shimizu A, Nagano J, Kawai A, Inoue A, Kagami T, Hirayama T, Yamaga A, Kaneda H, Kobayashi K, Minowa O, Miura I, Gondo Y, Noda T, Wakana S, Shiroishi T. Implementation of the modified-SHIRPA protocol for screening of dominant phenotypes in a large-scale ENU mutagenesis program. Mamm Genome. 2005 Nov;16(11):829-37. Epub 2005 Nov 11.
PubMed 16284798 Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome. 1997 Oct;8(10):711-3.
PubMed 9321461 Rogers DC, Jones DN, Nelson PR, Jones CM, Quilter CA, Robinson TL, Hagan JJ. Use of SHIRPA and discriminant analysis to characterise marked differences in the behavioural phenotype of six inbred mouse strains. Behav Brain Res. 1999 Nov 15;105(2):207-17.
PubMed 10563494 Rogers DC, Peters J, Martin JE, Ball S, Nicholson SJ, Witherden AS, Hafezparast M, Latcham J, Robinson TL, Quilter CA, Fisher EM. SHIRPA, a protocol for behavioral assessment: validation for longitudinal study of neurological dysfunction in mice. Neurosci Lett. 2001 Jun 22;306(1-2):89-92.
PubMed 11403965